

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM350324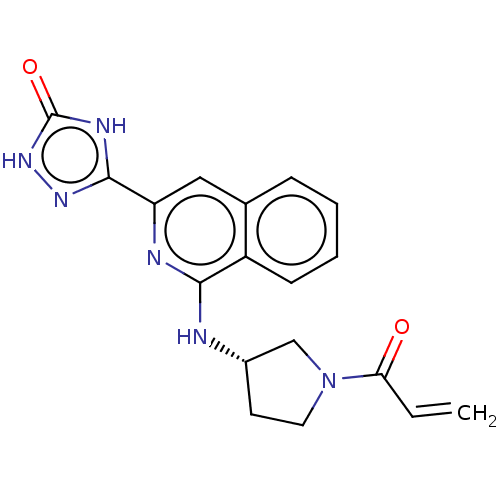 ((S)-3-(1-((1-acryloylpyrrolidin-3-yl)amino)isoquin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m... | Bioorg Med Chem Lett 19: 1600-3 (2009) BindingDB Entry DOI: 10.7270/Q2TQ63WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM350324 ((S)-3-(1-((1-acryloylpyrrolidin-3-yl)amino)isoquin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m... | US Patent US9801872 (2017) BindingDB Entry DOI: 10.7270/Q2BC41PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||