

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50123456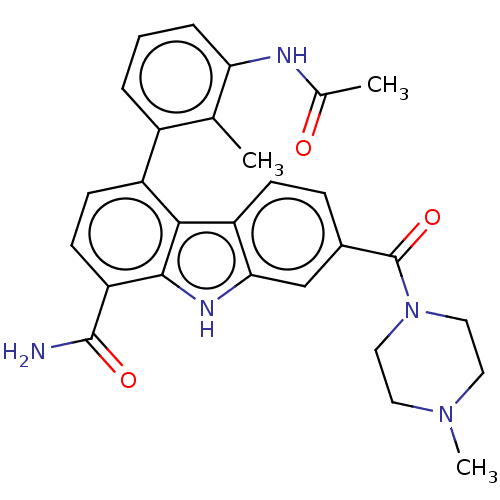 (CHEMBL3621515 | US10266491, Comparative Example 10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant BTK | Bioorg Med Chem Lett 25: 4265-9 (2015) Article DOI: 10.1016/j.bmcl.2015.07.102 BindingDB Entry DOI: 10.7270/Q2251M17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50123456 (CHEMBL3621515 | US10266491, Comparative Example 10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | J Med Chem 49: 4116-26 (2006) BindingDB Entry DOI: 10.7270/Q26112M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50123456 (CHEMBL3621515 | US10266491, Comparative Example 10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | J Med Chem 49: 4116-26 (2006) BindingDB Entry DOI: 10.7270/Q26112M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50123456 (CHEMBL3621515 | US10266491, Comparative Example 10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 ATP (20 and... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HT2SG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50123456 (CHEMBL3621515 | US10266491, Comparative Example 10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description A test compound was incubated with human recombinant Btk (100 nM) for 1.5 h at a concentration of 25 times the IC50 of Btk inhibition or 200 nM (whic... | US Patent US10676434 (2020) BindingDB Entry DOI: 10.7270/Q2CN76Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50123456 (CHEMBL3621515 | US10266491, Comparative Example 10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 ATP (20 and... | Citation and Details BindingDB Entry DOI: 10.7270/Q2HT2SG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50123456 (CHEMBL3621515 | US10266491, Comparative Example 10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US10676434 (2020) BindingDB Entry DOI: 10.7270/Q2CN76Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||