

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112931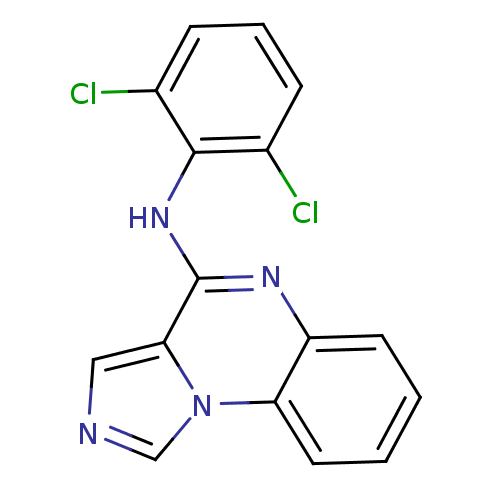 ((2,6-Dichloro-phenyl)-imidazo[1,5-a]quinoxalin-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against recombinant p56 Lck tyrosine kinase expressed as a His-tagged protein in insect cells using a baculovirus expression syst... | Bioorg Med Chem Lett 12: 1361-4 (2002) BindingDB Entry DOI: 10.7270/Q2R78DJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112931 ((2,6-Dichloro-phenyl)-imidazo[1,5-a]quinoxalin-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich Curated by ChEMBL | Assay Description Inhibition of Lck | J Med Chem 51: 1179-88 (2008) Article DOI: 10.1021/jm070654j BindingDB Entry DOI: 10.7270/Q29Z95RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112931 ((2,6-Dichloro-phenyl)-imidazo[1,5-a]quinoxalin-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Evaluated for inhibition of human p56 Lck tyrosine kinase | J Med Chem 47: 2534-49 (2004) Article DOI: 10.1021/jm0304358 BindingDB Entry DOI: 10.7270/Q2KH0P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||