 Found 3 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein kinase Mer' and Ligand = 'BDBM50055498'
Found 3 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein kinase Mer' and Ligand = 'BDBM50055498' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50055498
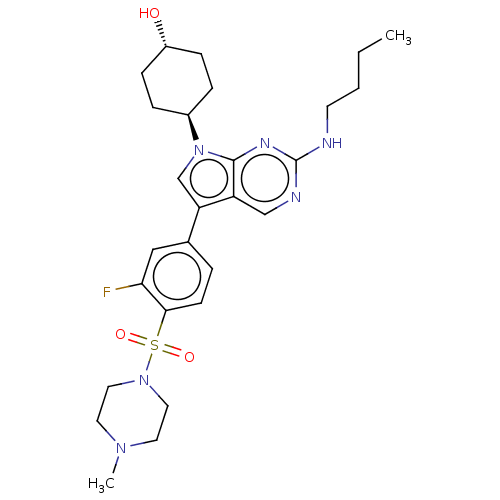
(CHEMBL3326004 | US10004755, Compound UNC1669A | US...)Show SMILES CCCCNc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)-c1ccc(c(F)c1)S(=O)(=O)N1CCN(C)CC1 |r,wU:12.11,wD:15.15,(-3.17,-12.13,;-1.85,-11.36,;-.52,-12.13,;.83,-11.36,;2.16,-12.13,;3.49,-11.36,;3.49,-9.82,;4.82,-9.05,;6.15,-9.81,;7.63,-9.33,;8.54,-10.59,;7.63,-11.84,;8.11,-13.31,;9.61,-13.63,;10.08,-15.1,;9.05,-16.24,;9.52,-17.7,;7.54,-15.91,;7.07,-14.45,;6.16,-11.36,;4.82,-12.13,;8.11,-7.87,;9.62,-7.55,;10.09,-6.1,;9.07,-4.95,;7.56,-5.27,;6.54,-4.12,;7.08,-6.73,;9.55,-3.48,;8.06,-3.08,;9.15,-1.99,;11.06,-3.18,;12.07,-4.34,;13.57,-4.04,;14.07,-2.59,;15.58,-2.29,;13.05,-1.43,;11.54,-1.73,)| Show InChI InChI=1S/C27H37FN6O3S/c1-3-4-11-29-27-30-17-22-23(18-34(26(22)31-27)20-6-8-21(35)9-7-20)19-5-10-25(24(28)16-19)38(36,37)33-14-12-32(2)13-15-33/h5,10,16-18,20-21,35H,3-4,6-9,11-15H2,1-2H3,(H,29,30,31)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer (unknown origin) by Off-chip Mobility Shift Assay |
J Med Chem 57: 7031-41 (2014)
Article DOI: 10.1021/jm500749d
BindingDB Entry DOI: 10.7270/Q2K075XQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50055498

(CHEMBL3326004 | US10004755, Compound UNC1669A | US...)Show SMILES CCCCNc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)-c1ccc(c(F)c1)S(=O)(=O)N1CCN(C)CC1 |r,wU:12.11,wD:15.15,(-3.17,-12.13,;-1.85,-11.36,;-.52,-12.13,;.83,-11.36,;2.16,-12.13,;3.49,-11.36,;3.49,-9.82,;4.82,-9.05,;6.15,-9.81,;7.63,-9.33,;8.54,-10.59,;7.63,-11.84,;8.11,-13.31,;9.61,-13.63,;10.08,-15.1,;9.05,-16.24,;9.52,-17.7,;7.54,-15.91,;7.07,-14.45,;6.16,-11.36,;4.82,-12.13,;8.11,-7.87,;9.62,-7.55,;10.09,-6.1,;9.07,-4.95,;7.56,-5.27,;6.54,-4.12,;7.08,-6.73,;9.55,-3.48,;8.06,-3.08,;9.15,-1.99,;11.06,-3.18,;12.07,-4.34,;13.57,-4.04,;14.07,-2.59,;15.58,-2.29,;13.05,-1.43,;11.54,-1.73,)| Show InChI InChI=1S/C27H37FN6O3S/c1-3-4-11-29-27-30-17-22-23(18-34(26(22)31-27)20-6-8-21(35)9-7-20)19-5-10-25(24(28)16-19)38(36,37)33-14-12-32(2)13-15-33/h5,10,16-18,20-21,35H,3-4,6-9,11-15H2,1-2H3,(H,29,30,31)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
| Assay Description
Inhibition constants of MerTK, Flt3, Tyro3 and Axl kinase activity by an active compound as described herein is determined at the Km for ATP using a ... |
Bioorg Med Chem 17: 7884-93 (2009)
BindingDB Entry DOI: 10.7270/Q26H4KR2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50055498

(CHEMBL3326004 | US10004755, Compound UNC1669A | US...)Show SMILES CCCCNc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)-c1ccc(c(F)c1)S(=O)(=O)N1CCN(C)CC1 |r,wU:12.11,wD:15.15,(-3.17,-12.13,;-1.85,-11.36,;-.52,-12.13,;.83,-11.36,;2.16,-12.13,;3.49,-11.36,;3.49,-9.82,;4.82,-9.05,;6.15,-9.81,;7.63,-9.33,;8.54,-10.59,;7.63,-11.84,;8.11,-13.31,;9.61,-13.63,;10.08,-15.1,;9.05,-16.24,;9.52,-17.7,;7.54,-15.91,;7.07,-14.45,;6.16,-11.36,;4.82,-12.13,;8.11,-7.87,;9.62,-7.55,;10.09,-6.1,;9.07,-4.95,;7.56,-5.27,;6.54,-4.12,;7.08,-6.73,;9.55,-3.48,;8.06,-3.08,;9.15,-1.99,;11.06,-3.18,;12.07,-4.34,;13.57,-4.04,;14.07,-2.59,;15.58,-2.29,;13.05,-1.43,;11.54,-1.73,)| Show InChI InChI=1S/C27H37FN6O3S/c1-3-4-11-29-27-30-17-22-23(18-34(26(22)31-27)20-6-8-21(35)9-7-20)19-5-10-25(24(28)16-19)38(36,37)33-14-12-32(2)13-15-33/h5,10,16-18,20-21,35H,3-4,6-9,11-15H2,1-2H3,(H,29,30,31)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill
US Patent
| Assay Description
The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... |
US Patent US9795606 (2017)
BindingDB Entry DOI: 10.7270/Q2FB553D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


