

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50076189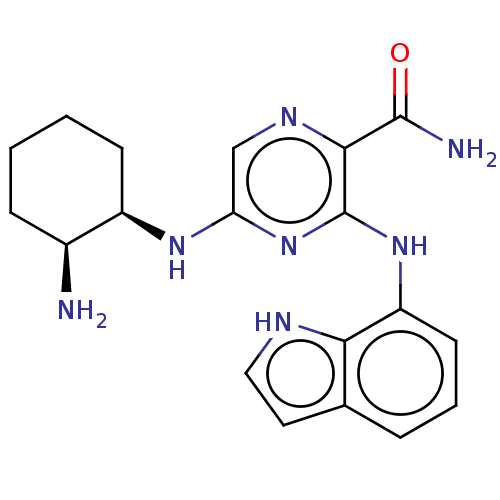 (CHEMBL3416024 | US9290481, 4.1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay | J Med Chem 58: 1950-63 (2015) Article DOI: 10.1021/jm5018863 BindingDB Entry DOI: 10.7270/Q2G44S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50076189 (CHEMBL3416024 | US9290481, 4.1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk in anti-igM stimulated human Ramos B cells assessed as phospho-BLNK level preincubated for 30 mins followed by anti-IgM stimulation... | J Med Chem 58: 1950-63 (2015) Article DOI: 10.1021/jm5018863 BindingDB Entry DOI: 10.7270/Q2G44S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50076189 (CHEMBL3416024 | US9290481, 4.1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 302 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk in anti-CD32 stimulated CD14+ human monocytes assessed as phospho-SLP76 level preincubated for 30 mins followed by anti-CD32 stimul... | J Med Chem 58: 1950-63 (2015) Article DOI: 10.1021/jm5018863 BindingDB Entry DOI: 10.7270/Q2G44S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||