

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50132436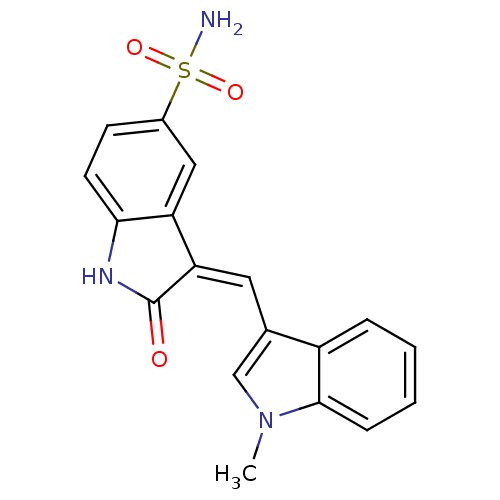 (3-((1-methyl-1H-indol-3-yl)methylene)-2-oxoindolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis | Bioorg Med Chem Lett 13: 3111-4 (2003) BindingDB Entry DOI: 10.7270/Q2ZG6SS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50132436 (3-((1-methyl-1H-indol-3-yl)methylene)-2-oxoindolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc. Curated by ChEMBL | Assay Description Inhibition of SYK | J Med Chem 55: 3614-43 (2012) Article DOI: 10.1021/jm201271b BindingDB Entry DOI: 10.7270/Q2NZ88RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Rattus norvegicus) | BDBM50132436 (3-((1-methyl-1H-indol-3-yl)methylene)-2-oxoindolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 313 | n/a | n/a | n/a | n/a |
Rigel, Inc. Curated by ChEMBL | Assay Description Inhibition of SYK in rat RBL2H3 cells assessed as reduction in DNP-BSA-stimulated Fcepsilon receptor 1-mediated 5-HT release | J Med Chem 55: 3614-43 (2012) Article DOI: 10.1021/jm201271b BindingDB Entry DOI: 10.7270/Q2NZ88RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||