 Found 6 hits Enz. Inhib. hit(s) with Target = 'Urokinase-type plasminogen activator' and Ligand = 'BDBM23891'
Found 6 hits Enz. Inhib. hit(s) with Target = 'Urokinase-type plasminogen activator' and Ligand = 'BDBM23891' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM23891
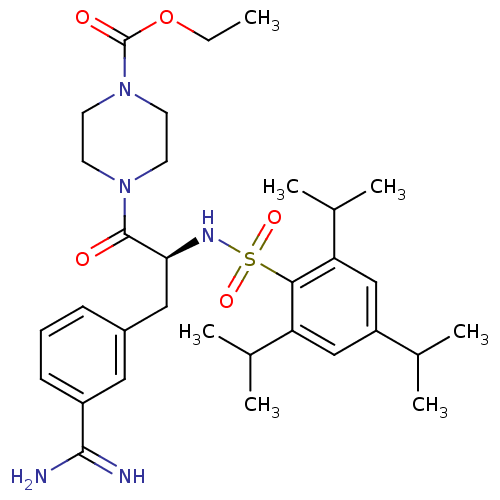
(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH
| Assay Description
The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... |
J Med Chem 49: 4116-26 (2006)
Article DOI: 10.1021/jm051272l
BindingDB Entry DOI: 10.7270/Q21C1V64 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM23891

(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Jena
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of plasminogen activator urokinase(microPa) |
Bioorg Med Chem Lett 9: 3147-52 (1999)
BindingDB Entry DOI: 10.7270/Q2RB73S3 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM23891

(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human uPA using pyroGlu-Gly-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins by spectrop... |
Bioorg Med Chem 20: 1557-68 (2012)
Article DOI: 10.1016/j.bmc.2011.12.040
BindingDB Entry DOI: 10.7270/Q2QR4XK1 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM23891

(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA)
Curated by ChEMBL
| Assay Description
Inhibition of uPA (unknown origin) |
J Med Chem 58: 9238-57 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01171
BindingDB Entry DOI: 10.7270/Q241713M |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM23891

(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of uPA (unknown origin) |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM23891

(3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O5S/c1-8-42-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34)35-43(40,41)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35H,8,12-15,17H2,1-7H3,(H3,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of uPA |
J Med Chem 51: 183-6 (2008)
Article DOI: 10.1021/jm701359z
BindingDB Entry DOI: 10.7270/Q2XG9QWK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data