

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893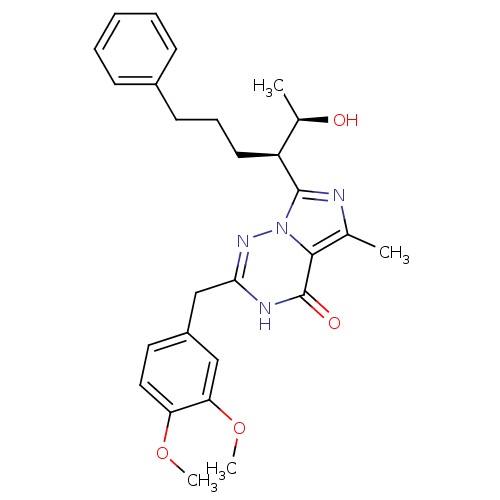 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 3626-3640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00116 BindingDB Entry DOI: 10.7270/Q2BV7K2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited , 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 7658-7676 (2017) Article DOI: 10.1021/acs.jmedchem.7b00709 BindingDB Entry DOI: 10.7270/Q2TT4TD5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 25 |
INTRA-CELLULAR THERAPIES, INC. US Patent | Assay Description Assay: PDE2A is assayed with FL-cAMP as substrate. An enzyme titration is first performed to determine the working concentration of PDE. The concentr... | US Patent US10105349 (2018) BindingDB Entry DOI: 10.7270/Q2QN68T1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC. US Patent | Assay Description r-hPDE2A (Accession No. NM_002599, Homo sapiens phosphodiesterase 2A, cGMP-stimulated, transcript variant 1) A mammalian expression cloning vector wi... | US Patent US10543194 (2020) BindingDB Entry DOI: 10.7270/Q2F47RJZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of PDE2a (unknown origin) using cAMP as substrate after 30 mins by Scintillation proximity assay | J Med Chem 60: 2037-2051 (2017) Article DOI: 10.1021/acs.jmedchem.6b01793 BindingDB Entry DOI: 10.7270/Q2Q242GF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of human His-tagged PDE2A catalytic domain (578 to 919 residues) expressed in Escherichia coli BL21-CodonPlus(DE3) cells using cGMP as sub... | J Med Chem 59: 7029-65 (2016) Article DOI: 10.1021/acs.jmedchem.5b01813 BindingDB Entry DOI: 10.7270/Q2RX9GJ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Changzhou University Curated by ChEMBL | Assay Description Inhibition of PDE2 (unknown origin) using [3H]cAMP as substrate measured after 30 mins by scintillation proximity assay | Bioorg Med Chem Lett 29: 481-486 (2019) Article DOI: 10.1016/j.bmcl.2018.12.018 BindingDB Entry DOI: 10.7270/Q2SB4947 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of human His-tagged PDE2A catalytic domain (578 to 919 residues) expressed in Escherichia coli BL21-CodonPlus(DE3) cells using cGMP as sub... | J Med Chem 59: 7029-65 (2016) Article DOI: 10.1021/acs.jmedchem.5b01813 BindingDB Entry DOI: 10.7270/Q2RX9GJ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 2 | J Med Chem 48: 3449-62 (2005) Article DOI: 10.1021/jm040217u BindingDB Entry DOI: 10.7270/Q21G0N2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Isolated cardiomyocytes were washed twice in PBS at room temperature, transferred to 4% paraformaldehyde (PFA) and gently shaken for 30 min, then was... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CR5XK9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) using [3H]cGMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 60: 7677-7702 (2017) Article DOI: 10.1021/acs.jmedchem.7b00807 BindingDB Entry DOI: 10.7270/Q2P271JR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Changzhou University Curated by ChEMBL | Assay Description Inhibition of PDE2 (unknown origin) using [3H]cAMP or [3H]cGMP as substrate measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem Lett 29: 481-486 (2019) Article DOI: 10.1016/j.bmcl.2018.12.018 BindingDB Entry DOI: 10.7270/Q2SB4947 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||