 Found 5 hits of kd for drug = Amcill
Found 5 hits of kd for drug = Amcill Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM18137
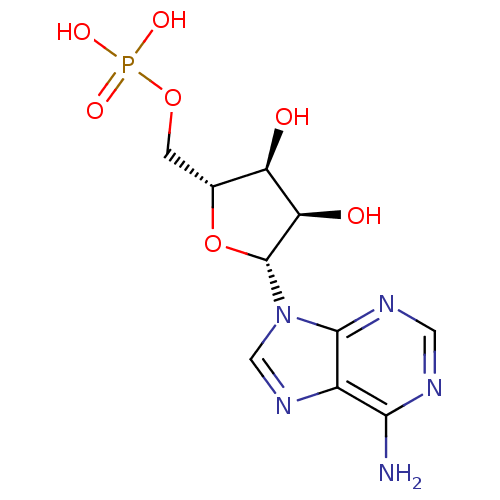
(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | 1.13 | n/a | n/a | 4.43E+6 | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Association rate constant for the interaction between inhibitor and HIV-1 protease |
J Med Chem 45: 5430-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GH9JP4 |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-1/subunit gamma-1
(Homo sapiens (Human)) | BDBM18137

(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human AMPK alpha1/beta1/gamma1 by SPR binding assay |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM18137

(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human biotinylated N-terminal GST-tagged autophosphorylated TAK1 (1 to 303 residues) fused with TAB1 (437 to 504 resi... |
Bioorg Med Chem Lett 27: 1031-1036 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.064
BindingDB Entry DOI: 10.7270/Q22J6F3R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM18137

(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.70E+5 | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human biotinylated N-terminal GST-tagged non-autophosphorylated TAK1 (1 to 303 residues) fused with TAB1 (437 to 504 ... |
Bioorg Med Chem Lett 27: 1031-1036 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.064
BindingDB Entry DOI: 10.7270/Q22J6F3R |
More data for this
Ligand-Target Pair | |
2-dehydropantoate 2-reductase
(Escherichia coli (strain K12)) | BDBM18137

(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a |
University Chemical Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity to Escherichia coli KPR |
J Med Chem 49: 4992-5000 (2006)
Article DOI: 10.1021/jm060490r
BindingDB Entry DOI: 10.7270/Q28S4QQN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data