 Found 13 hits of ki for drug = Alimta
Found 13 hits of ki for drug = Alimta Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18796
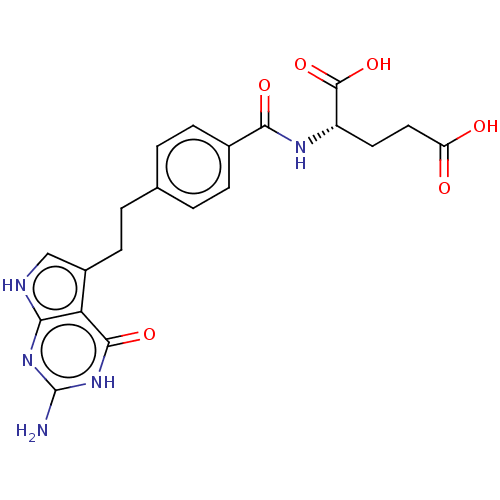
((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Theoretical Studies gGmbH
Curated by ChEMBL
| Assay Description
Inhibition of thymidin synthase |
J Med Chem 53: 6539-49 (2010)
Article DOI: 10.1021/jm901869w
BindingDB Entry DOI: 10.7270/Q24J0F9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human dihydrofolate reductase done at 37 degree C in pH 7.4 with compound |
J Med Chem 48: 5329-36 (2005)
Article DOI: 10.1021/jm058213s
BindingDB Entry DOI: 10.7270/Q2RV0PF5 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Theoretical Studies gGmbH
Curated by ChEMBL
| Assay Description
Inhibition of DHFR |
J Med Chem 53: 6539-49 (2010)
Article DOI: 10.1021/jm901869w
BindingDB Entry DOI: 10.7270/Q24J0F9S |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 5.5 by Dixon plot |
J Med Chem 55: 1758-70 (2012)
Article DOI: 10.1021/jm201688n
BindingDB Entry DOI: 10.7270/Q26Q1ZB5 |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Displacement of [3H]MTX from human PCFT expressed in Chinese hamster R2 cells at pH 5.5 by Dixon plot |
J Med Chem 53: 1306-18 (2010)
Article DOI: 10.1021/jm9015729
BindingDB Entry DOI: 10.7270/Q23N24B0 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthase |
J Med Chem 35: 4450-4 (1992)
BindingDB Entry DOI: 10.7270/Q2RR1ZV6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthetase at 37 degree C pH 7.4 |
J Med Chem 48: 5329-36 (2005)
Article DOI: 10.1021/jm058213s
BindingDB Entry DOI: 10.7270/Q2RV0PF5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Trifunctional purine biosynthetic protein adenosine-3
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Theoretical Studies gGmbH
Curated by ChEMBL
| Assay Description
Inhibition of GARFT |
J Med Chem 53: 6539-49 (2010)
Article DOI: 10.1021/jm901869w
BindingDB Entry DOI: 10.7270/Q24J0F9S |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Displacement of [3H]MTX from human PCFT expressed in Chinese hamster R2 cells at pH 6.8 by Dixon plot |
J Med Chem 53: 1306-18 (2010)
Article DOI: 10.1021/jm9015729
BindingDB Entry DOI: 10.7270/Q23N24B0 |
More data for this
Ligand-Target Pair | |
Proton-coupled folate transporter
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MTX transport at human PCFT expressed in Chinese hamster R2 cells at pH 6.8 by Dixon plot |
J Med Chem 55: 1758-70 (2012)
Article DOI: 10.1021/jm201688n
BindingDB Entry DOI: 10.7270/Q26Q1ZB5 |
More data for this
Ligand-Target Pair | |
Serine hydroxymethyltransferase, cytosolic
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant Serine hydroxymethyltransferase, cytosolic by spectrophotometry |
Eur J Med Chem 46: 1616-21 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.009
BindingDB Entry DOI: 10.7270/Q22V2H35 |
More data for this
Ligand-Target Pair | |
Serine hydroxymethyltransferase, cytosolic
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Serine hydroxymethyltransferase, cytosolic measured by slope intercepts of mixed-type inhibition against compound con... |
Eur J Med Chem 46: 1616-21 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.009
BindingDB Entry DOI: 10.7270/Q22V2H35 |
More data for this
Ligand-Target Pair | |
Serine hydroxymethyltransferase, cytosolic
(Homo sapiens (Human)) | BDBM18796

((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human recombinant Serine hydroxymethyltransferase, cytosolic measured by Y-axes intercepts of mixed-type inhibition against ... |
Eur J Med Chem 46: 1616-21 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.009
BindingDB Entry DOI: 10.7270/Q22V2H35 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data