

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM52947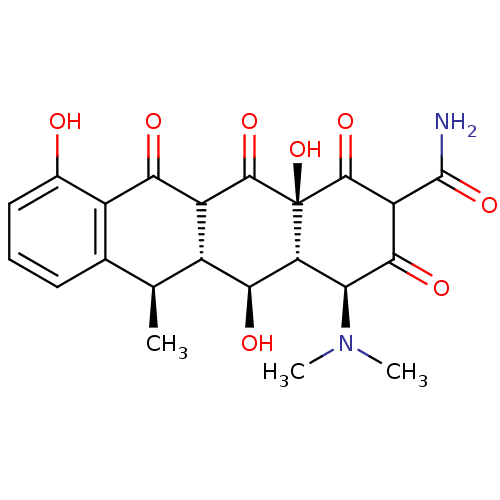 ((2Z,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2BZ64H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM52947 ((2Z,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q26M35BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2-microglobulin (Homo sapiens (Human)) | BDBM52947 ((2Z,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Pavia | Assay Description Inhibition of wild type beta-m fibrillogenesis tetracycline congeners and was follow by thioflavin fluorescence assay. | J Biol Chem 286: 2121-31 (2011) Article DOI: 10.1074/jbc.M110.178376 BindingDB Entry DOI: 10.7270/Q2542M6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM52947 ((2Z,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of PAD4 by ABPP-based assay | Bioorg Med Chem 16: 739-45 (2008) Article DOI: 10.1016/j.bmc.2007.10.021 BindingDB Entry DOI: 10.7270/Q24F1RKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||