

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50293305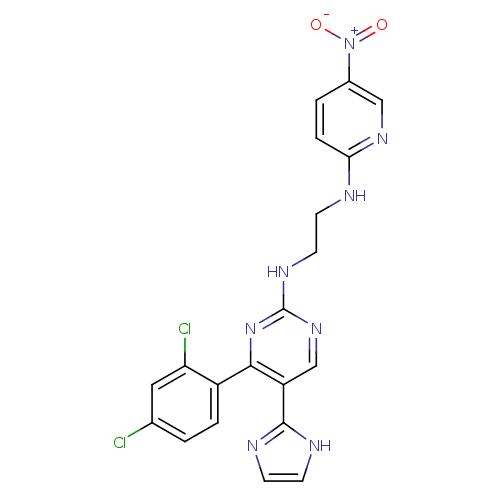 (4-(2,4-dichlorophenyl)-5-(1H-imidazol-2-yl)-N-(2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road Curated by ChEMBL | Assay Description Inhibition of human GSK3beta | Eur J Med Chem 44: 2361-71 (2009) Article DOI: 10.1016/j.ejmech.2008.08.012 BindingDB Entry DOI: 10.7270/Q2057FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50397757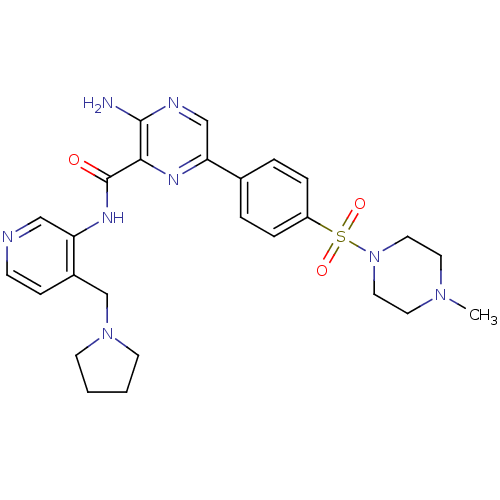 (CHEMBL2177173) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... | J Med Chem 55: 9107-19 (2012) Article DOI: 10.1021/jm201724m BindingDB Entry DOI: 10.7270/Q2X0685T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8143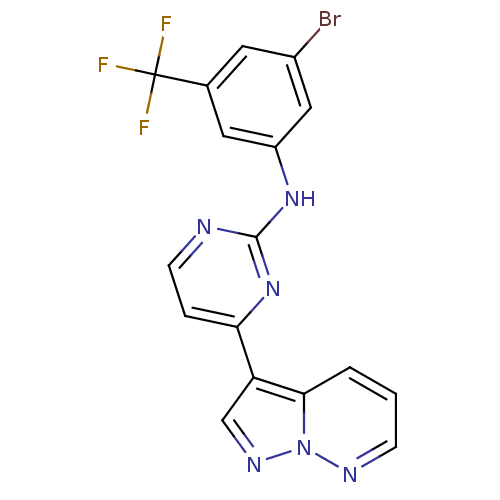 (N-[3-bromo-5-(trifluoromethyl)phenyl]-4-{pyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313057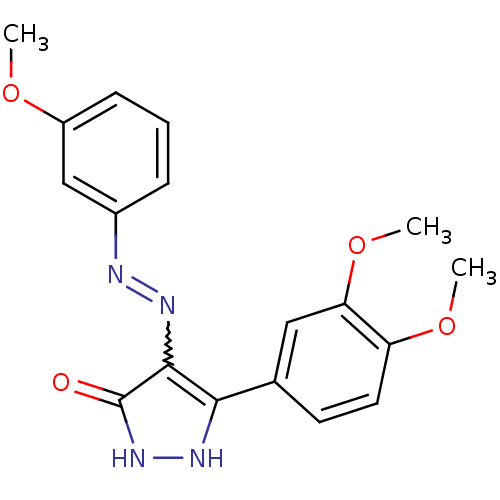 ((Z)-3-(3,4-dimethoxyphenyl)-4-(2-(3-methoxyphenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of GSK3-beta assessed as NADH level after 10 mins by pyruvate kinase/lactate dehydrogenase coupled spectrophotometric assay | Bioorg Med Chem Lett 20: 1661-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.072 BindingDB Entry DOI: 10.7270/Q2TX3G9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313056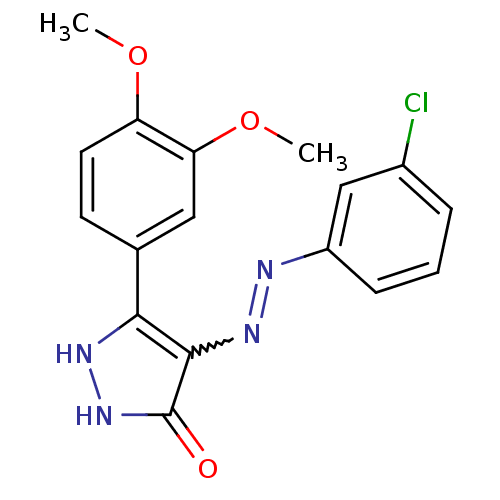 ((Z)-4-(2-(3-chlorophenyl)hydrazono)-3-(3,4-dimetho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of GSK3-beta assessed as NADH level after 10 mins by pyruvate kinase/lactate dehydrogenase coupled spectrophotometric assay | Bioorg Med Chem Lett 20: 1661-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.072 BindingDB Entry DOI: 10.7270/Q2TX3G9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50397744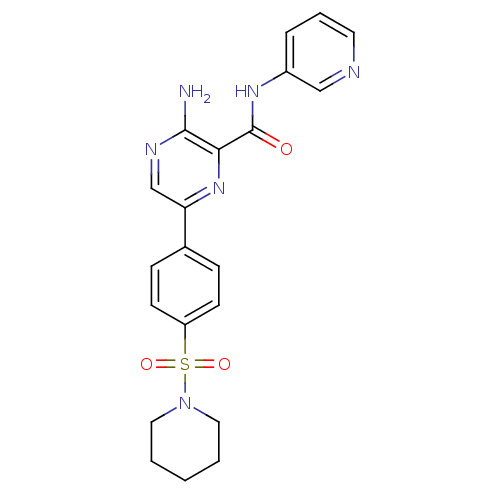 (CHEMBL2177163) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... | J Med Chem 55: 9107-19 (2012) Article DOI: 10.1021/jm201724m BindingDB Entry DOI: 10.7270/Q2X0685T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50397749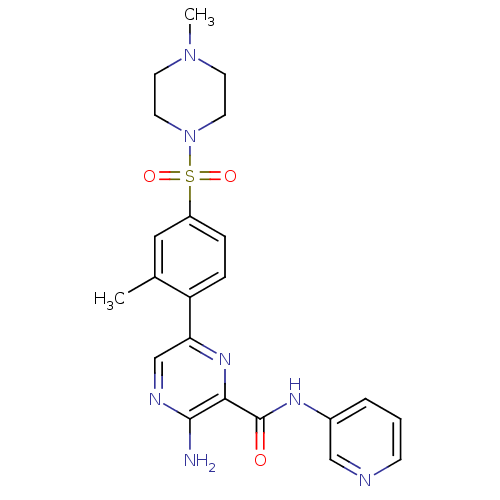 (CHEMBL2182002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... | J Med Chem 55: 9107-19 (2012) Article DOI: 10.1021/jm201724m BindingDB Entry DOI: 10.7270/Q2X0685T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50397756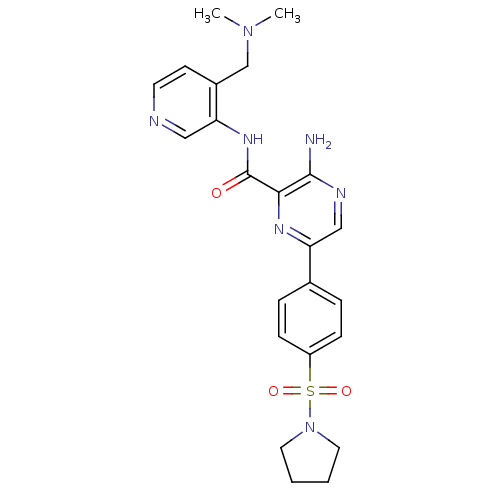 (CHEMBL2177174) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... | J Med Chem 55: 9107-19 (2012) Article DOI: 10.1021/jm201724m BindingDB Entry DOI: 10.7270/Q2X0685T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50397743 (CHEMBL2177165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... | J Med Chem 55: 9107-19 (2012) Article DOI: 10.1021/jm201724m BindingDB Entry DOI: 10.7270/Q2X0685T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498332 (US11014911, Example 157 | US11014911, Example 158 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His6-tagged human recombinant GSK3beta H350L mutant expressed in baculovirus-infected Sf21 cells assessed as reduction in pr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00500 BindingDB Entry DOI: 10.7270/Q29G5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498332 (US11014911, Example 157 | US11014911, Example 158 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of GSK3β assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluorescenc... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498332 (US11014911, Example 157 | US11014911, Example 158 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313050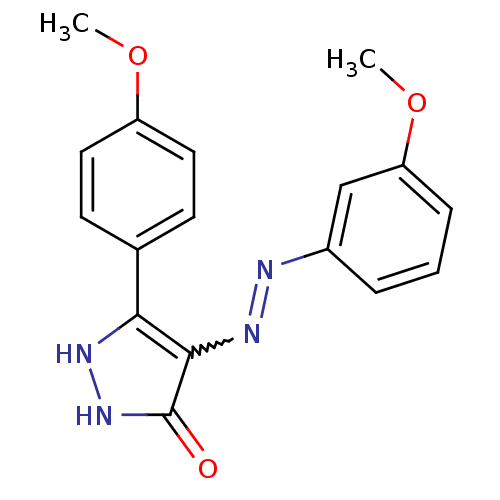 ((Z)-3-(4-methoxyphenyl)-4-(2-(3-methoxyphenyl)hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of GSK3-beta assessed as NADH level after 10 mins by pyruvate kinase/lactate dehydrogenase coupled spectrophotometric assay | Bioorg Med Chem Lett 20: 1661-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.072 BindingDB Entry DOI: 10.7270/Q2TX3G9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498320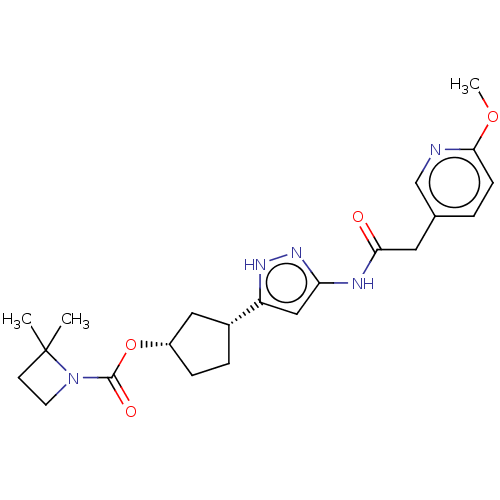 (US11014911, Example 145 | US11718603, Example 145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of GSK3β assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluorescenc... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498320 (US11014911, Example 145 | US11718603, Example 145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498320 (US11014911, Example 145 | US11718603, Example 145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His6-tagged human recombinant GSK3beta H350L mutant expressed in baculovirus-infected Sf21 cells assessed as reduction in pr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00500 BindingDB Entry DOI: 10.7270/Q29G5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50397753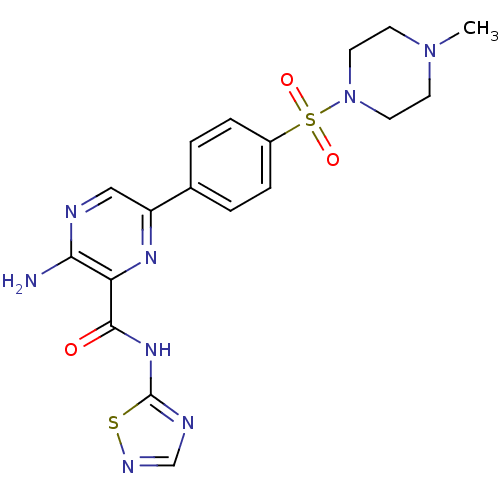 (CHEMBL2177177) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... | J Med Chem 55: 9107-19 (2012) Article DOI: 10.1021/jm201724m BindingDB Entry DOI: 10.7270/Q2X0685T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8145 (N-(3,5-dichlorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8138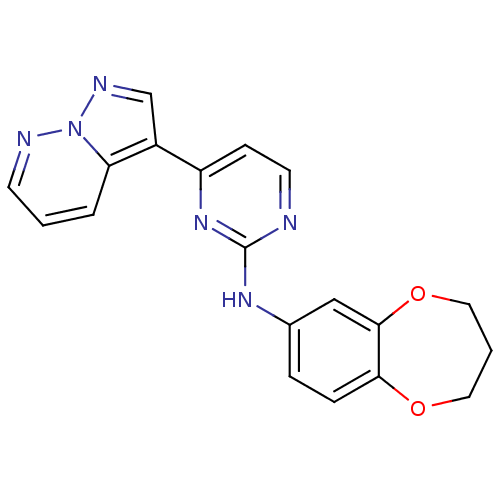 (N-(3,4-dihydro-2H-1,5-benzodioxepin-7-yl)-4-{pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50397748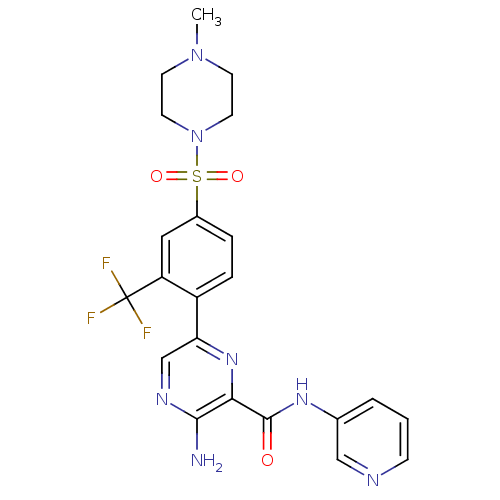 (CHEMBL2177155) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... | J Med Chem 55: 9107-19 (2012) Article DOI: 10.1021/jm201724m BindingDB Entry DOI: 10.7270/Q2X0685T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498319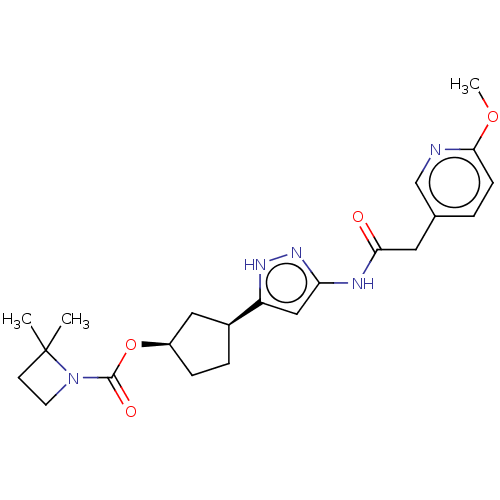 (US11014911, Example 144 | US11718603, Example 144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of GSK3β assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluorescenc... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498319 (US11014911, Example 144 | US11718603, Example 144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50397768 (CHEMBL2182001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... | J Med Chem 55: 9107-19 (2012) Article DOI: 10.1021/jm201724m BindingDB Entry DOI: 10.7270/Q2X0685T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498319 (US11014911, Example 144 | US11718603, Example 144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His6-tagged human recombinant GSK3beta H350L mutant expressed in baculovirus-infected Sf21 cells assessed as reduction in pr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00500 BindingDB Entry DOI: 10.7270/Q29G5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498707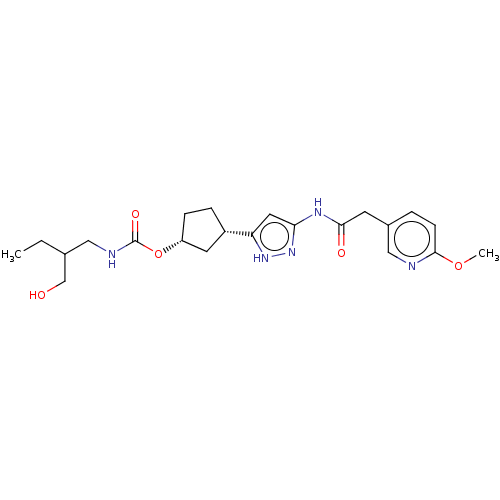 ((1R,3S)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His6-tagged human recombinant GSK3beta H350L mutant expressed in baculovirus-infected Sf21 cells assessed as reduction in pr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00500 BindingDB Entry DOI: 10.7270/Q29G5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498707 ((1R,3S)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of GSK3β assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluorescenc... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498707 ((1R,3S)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50397767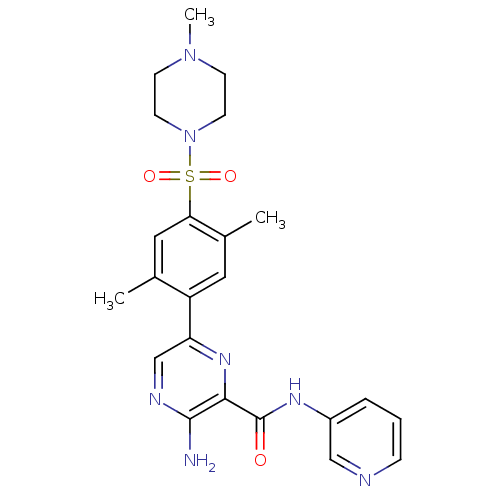 (CHEMBL2177156) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... | J Med Chem 55: 9107-19 (2012) Article DOI: 10.1021/jm201724m BindingDB Entry DOI: 10.7270/Q2X0685T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498330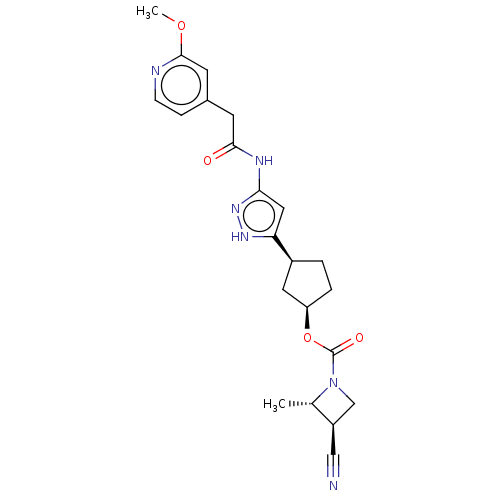 (US11014911, Example 155 | US11014911, Example 156 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of GSK3β assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluorescenc... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498330 (US11014911, Example 155 | US11014911, Example 156 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498334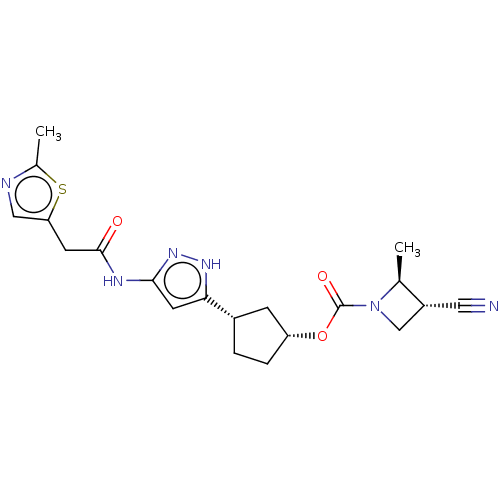 (US11014911, Example 159 | US11014911, Example 160 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His6-tagged human recombinant GSK3beta H350L mutant expressed in baculovirus-infected Sf21 cells assessed as reduction in pr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00500 BindingDB Entry DOI: 10.7270/Q29G5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498334 (US11014911, Example 159 | US11014911, Example 160 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of GSK3β assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluorescenc... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498334 (US11014911, Example 159 | US11014911, Example 160 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498731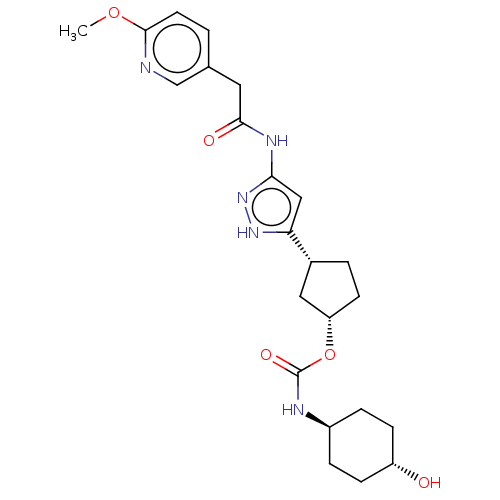 ((1S,3R)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498731 ((1S,3R)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of GSK3β assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluorescenc... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313048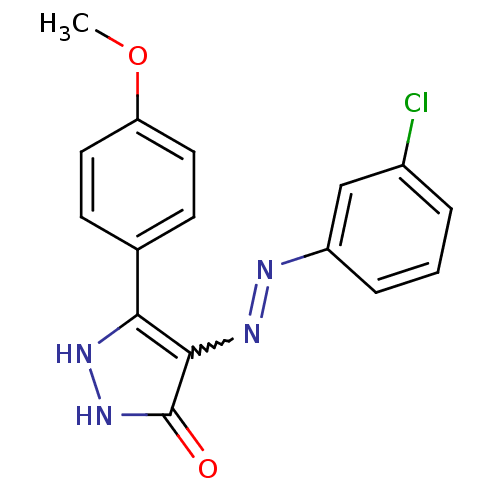 ((Z)-4-(2-(3-chlorophenyl)hydrazono)-3-(4-methoxyph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of GSK3-beta assessed as NADH level after 10 mins by pyruvate kinase/lactate dehydrogenase coupled spectrophotometric assay | Bioorg Med Chem Lett 20: 1661-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.072 BindingDB Entry DOI: 10.7270/Q2TX3G9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498728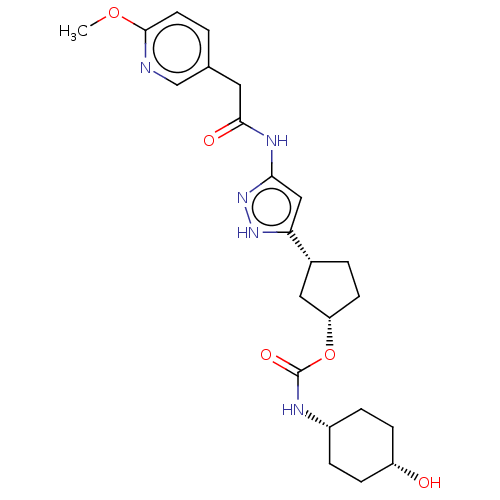 ((1S,3R)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498731 ((1S,3R)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His6-tagged human recombinant GSK3beta H350L mutant expressed in baculovirus-infected Sf21 cells assessed as reduction in pr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00500 BindingDB Entry DOI: 10.7270/Q29G5RDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498728 ((1S,3R)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of GSK3β assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluorescenc... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498719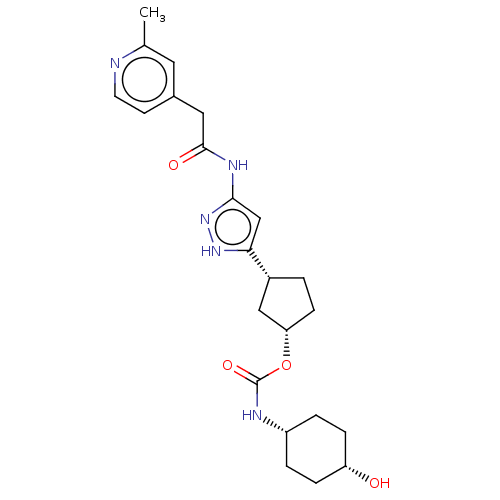 ((1S,3R)-3-(3-{[(2- methylpyridin-4- yl)acetyl]am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7SJ2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM498719 ((1S,3R)-3-(3-{[(2- methylpyridin-4- yl)acetyl]am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The purpose of GSK3β assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) of small molecule inhibitors by using a fluorescenc... | US Patent US11014911 (2021) BindingDB Entry DOI: 10.7270/Q23N26H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8142 (N-[3-methoxy-5-(trifluoromethyl)phenyl]-4-{pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8140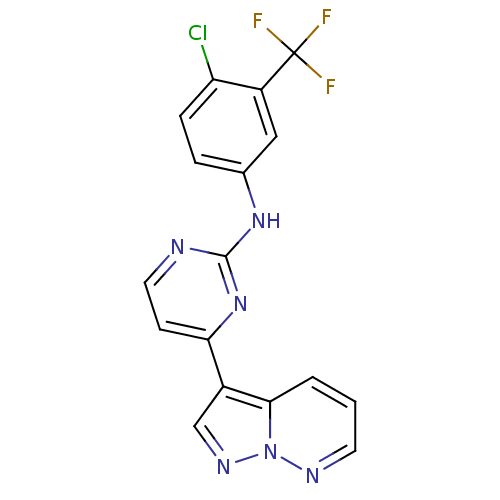 (N-[4-chloro-3-(trifluoromethyl)phenyl]-4-{pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8129 (4-[(4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidin-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8171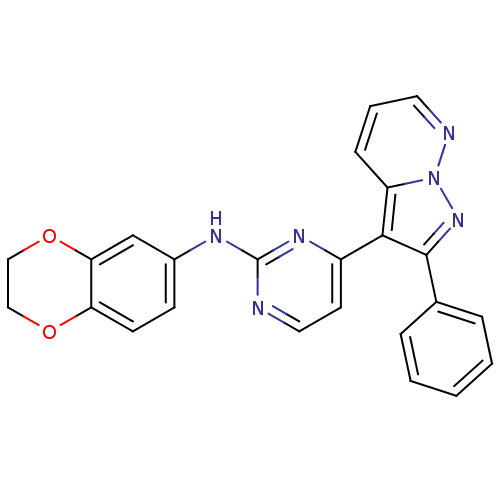 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{2-phenylpy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8130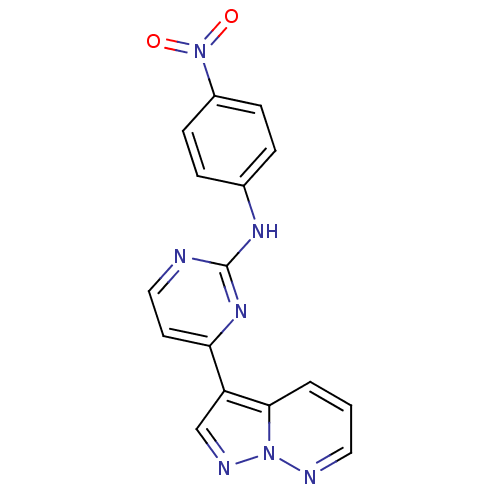 (N-(4-nitrophenyl)-4-{pyrazolo[1,5-a]pyridazin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8144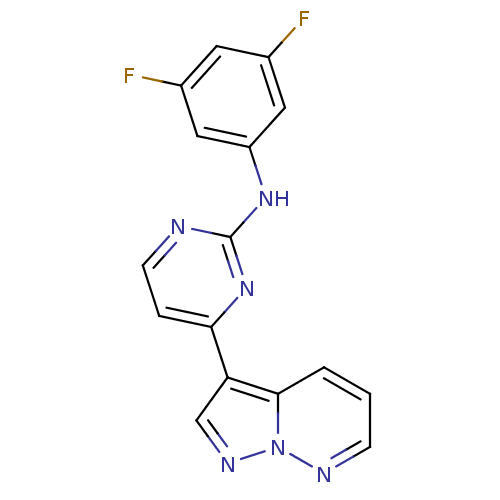 (N-(3,5-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8135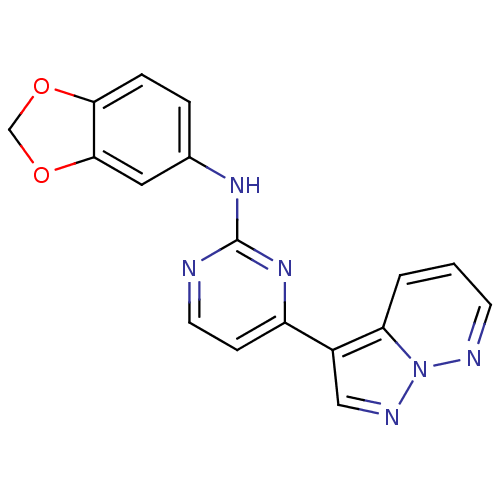 (N-(2H-1,3-benzodioxol-5-yl)-4-{pyrazolo[1,5-a]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1040 total ) | Next | Last >> |