

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50044561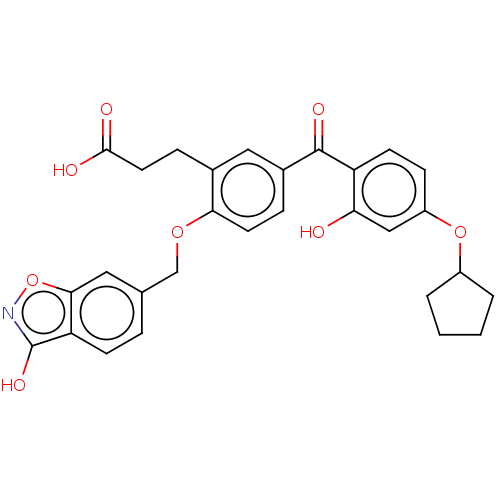 (CHEMBL3222137) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Substrate inhibition of human UGT1A1-mediated T-5224 acyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC meth... | Drug Metab Dispos 39: 803-13 (2011) Article DOI: 10.1124/dmd.110.037952 BindingDB Entry DOI: 10.7270/Q2W37Z2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50044561 (CHEMBL3222137) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Substrate inhibition of human UGT1A1-mediated T-5224 hydroxyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC ... | Drug Metab Dispos 39: 803-13 (2011) Article DOI: 10.1124/dmd.110.037952 BindingDB Entry DOI: 10.7270/Q2W37Z2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50088502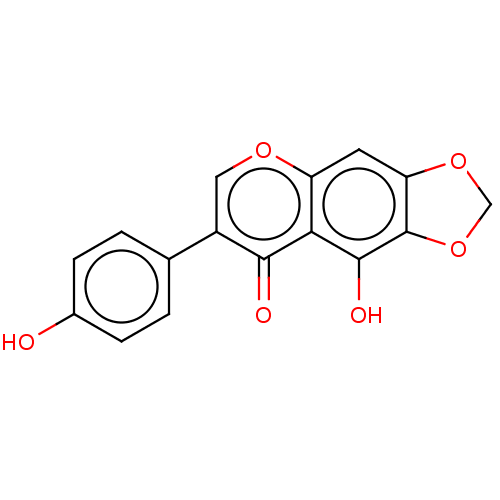 (CHEBI:5970 | CHEMBL3527329) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Rubner-Institute Curated by ChEMBL | Assay Description Substrate inhibition of human recombinant UGT1A1 assessed as IRI-O-5-monoglucuronide formation incubated for 5 mins prior to UDPGA addition measured ... | Drug Metab Dispos 39: 610-6 (2011) Article DOI: 10.1124/dmd.110.033076 BindingDB Entry DOI: 10.7270/Q20V8FHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50088502 (CHEBI:5970 | CHEMBL3527329) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Rubner-Institute Curated by ChEMBL | Assay Description Substrate inhibition of human recombinant UGT1A1 assessed as IRI-O-4'-monoglucuronide formation incubated for 5 mins prior to UDPGA addition measured... | Drug Metab Dispos 39: 610-6 (2011) Article DOI: 10.1124/dmd.110.033076 BindingDB Entry DOI: 10.7270/Q20V8FHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM23411 (5,6,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Drug metabolism assessed as human recombinant UGT1A1-mediated formation of scutellarein-7-O-glucuronide after 25 mins by HPLC/UV analysis | Drug Metab Dispos 40: 2009-20 (2012) Article DOI: 10.1124/dmd.112.047183 BindingDB Entry DOI: 10.7270/Q2V40WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM13066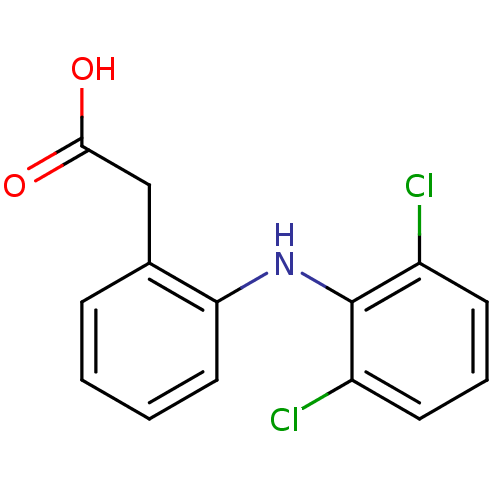 (2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of British Columbia Curated by ChEMBL | Assay Description Inhibition of 4-methylumbelliferone glucuronidation by human recombinant UGT1A1 | Pharmacol Ther 106: 97-132 (2005) Article DOI: 10.1016/j.pharmthera.2004.10.013 BindingDB Entry DOI: 10.7270/Q2959HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50242284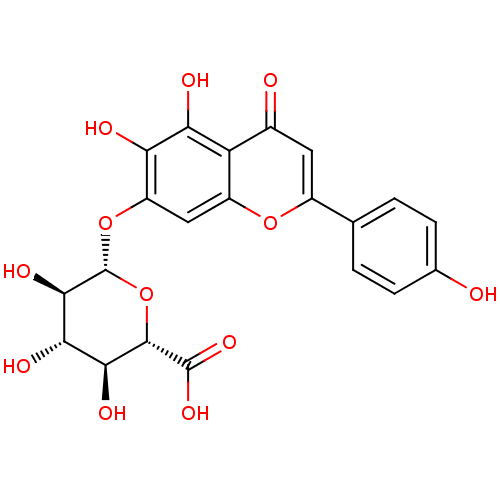 (CHEMBL487805 | scutellarin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 9.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Drug metabolism assessed as human recombinant UGT1A1-mediated formation of scutellarein-6,7-diglucuronide after 25 mins by HPLC/UV analysis | Drug Metab Dispos 40: 2009-20 (2012) Article DOI: 10.1124/dmd.112.047183 BindingDB Entry DOI: 10.7270/Q2V40WXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50206509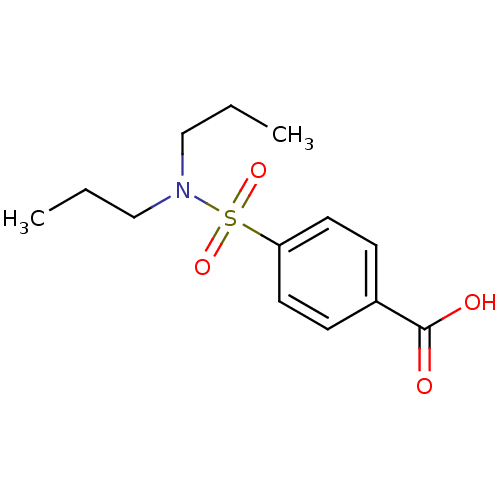 (4-Dipropylsulfamoyl-benzoic acid | 4-Dipropylsulfa...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of British Columbia Curated by ChEMBL | Assay Description Inhibition of 4-methylumbelliferone glucuronidation by human recombinant UGT1A1 | Pharmacol Ther 106: 97-132 (2005) Article DOI: 10.1016/j.pharmthera.2004.10.013 BindingDB Entry DOI: 10.7270/Q2959HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||