

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00632 BindingDB Entry DOI: 10.7270/Q2SB49SR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human UGT1A1 using bilirubin as substrate preincubated for 5 mins followed by substrate addition and measured after 40 mins... | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of UGT1A1 in human liver microsomes using beta-estradiol as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00632 BindingDB Entry DOI: 10.7270/Q2SB49SR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50068270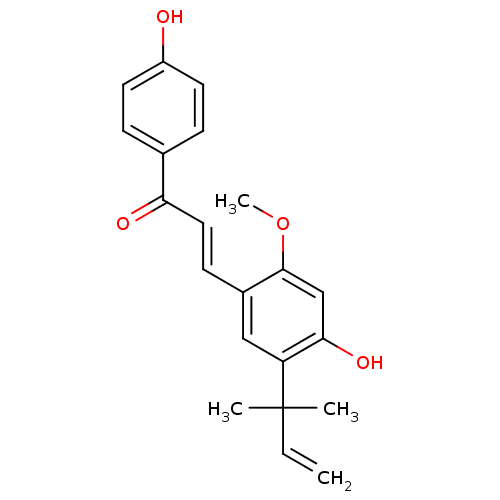 ((E)-3-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Induction of UGT1A1 (unknown origin) assessed as increase in intracellular acidic autophagy vesicles formation | J Med Chem 63: 10135-10157 (2020) Article DOI: 10.1021/acs.jmedchem.9b02038 BindingDB Entry DOI: 10.7270/Q2S46WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50599144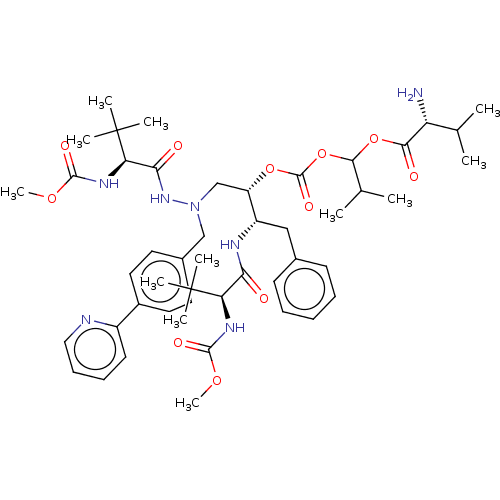 (CHEMBL5179012) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00632 BindingDB Entry DOI: 10.7270/Q2SB49SR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50460863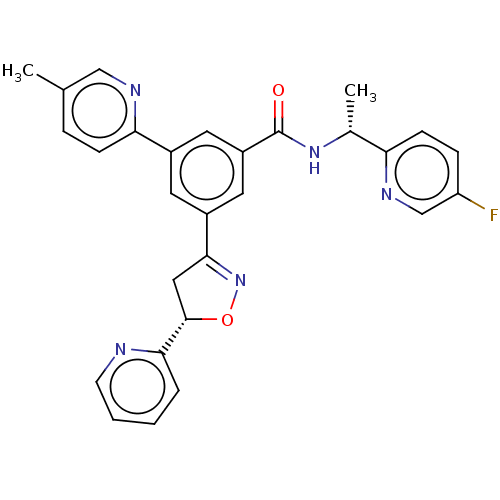 (CHEMBL4227228) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human UGT1A1 expressed in baculovirus infected insect cells using bilirubin as substrate measured after 10 mins by HPLC analysis | Bioorg Med Chem Lett 28: 1392-1396 (2018) Article DOI: 10.1016/j.bmcl.2018.02.039 BindingDB Entry DOI: 10.7270/Q25T3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50576021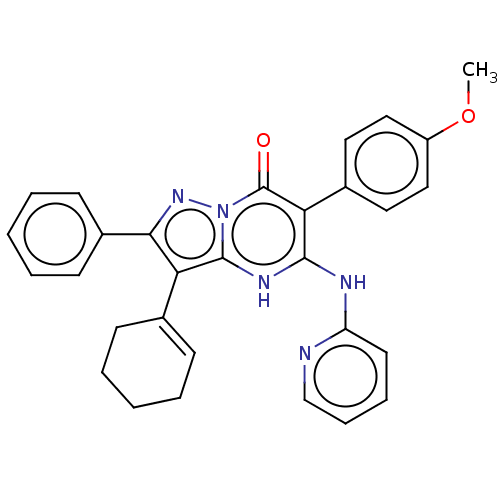 (CHEMBL4573938) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT1A1 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01895 BindingDB Entry DOI: 10.7270/Q20R9T7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50458163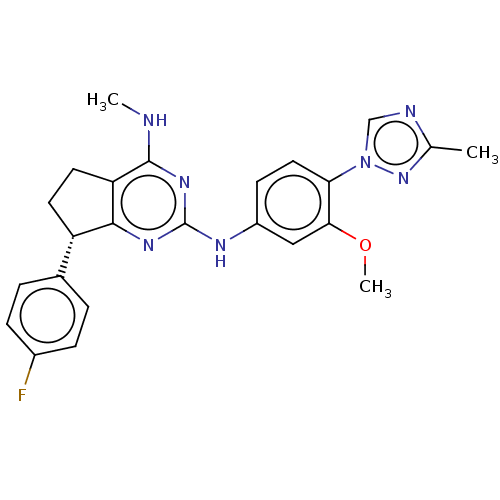 (CHEMBL4209316) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human UGT1A1 | Citation and Details Article DOI: 10.1021/acsmedchemlett.8b00541 BindingDB Entry DOI: 10.7270/Q24171P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50553286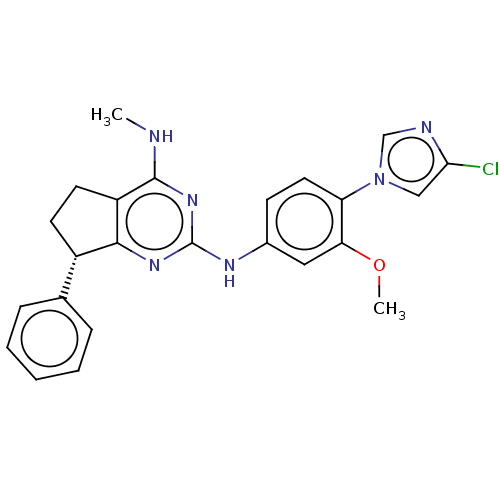 (CHEMBL4794465) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human UGT1A1 | Citation and Details Article DOI: 10.1021/acsmedchemlett.8b00541 BindingDB Entry DOI: 10.7270/Q24171P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50600733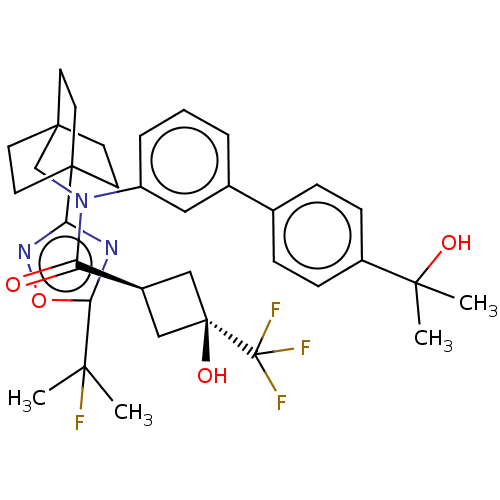 (CHEMBL5182534) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00165 BindingDB Entry DOI: 10.7270/Q27S7STN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50088504 (A-84538 | ABBOTT-84538 | CHEBI:45409 | Norvir | Ri...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50599144 (CHEMBL5179012) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00632 BindingDB Entry DOI: 10.7270/Q2SB49SR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50523559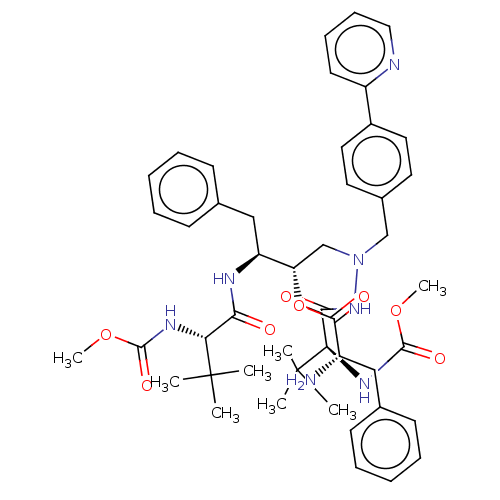 (CHEMBL4573907) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of UGT1A1 in human liver microsomes using beta-estradiol as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50523559 (CHEMBL4573907) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human UGT1A1 using bilirubin as substrate preincubated for 5 mins followed by substrate addition and measured after 40 mins... | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50088504 (A-84538 | ABBOTT-84538 | CHEBI:45409 | Norvir | Ri...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50101979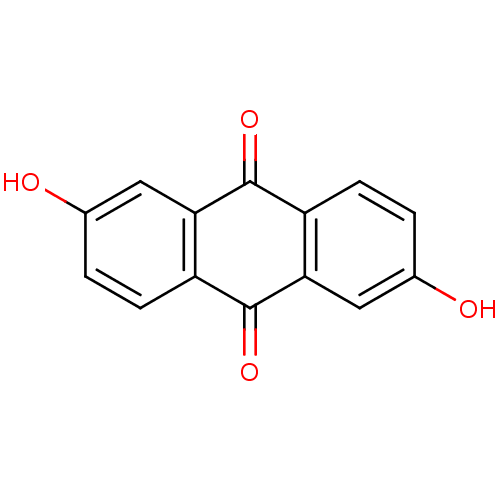 (2,6-Dihydroxyanthraquinone | 2,6-dihydroxy-9,10-an...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM372346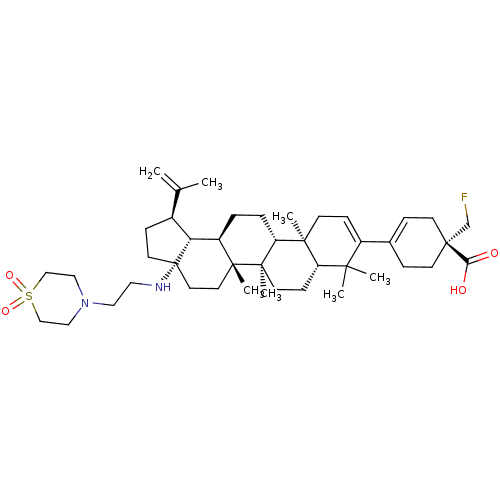 (Method A: (R)-4-((1R,3aS,5aR,5bR,7aR,11aS,11bR,13a...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00879 BindingDB Entry DOI: 10.7270/Q26W9G2J | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50101979 (2,6-Dihydroxyanthraquinone | 2,6-dihydroxy-9,10-an...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50301375 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50550263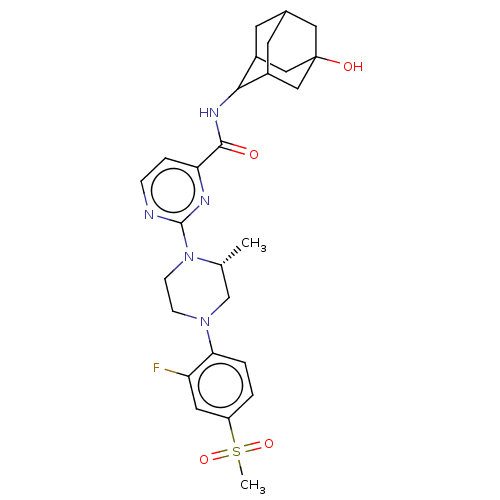 (CHEMBL4748750) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT1A1 (unknown origin) | Citation and Details BindingDB Entry DOI: 10.7270/Q27P930B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50301375 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM23420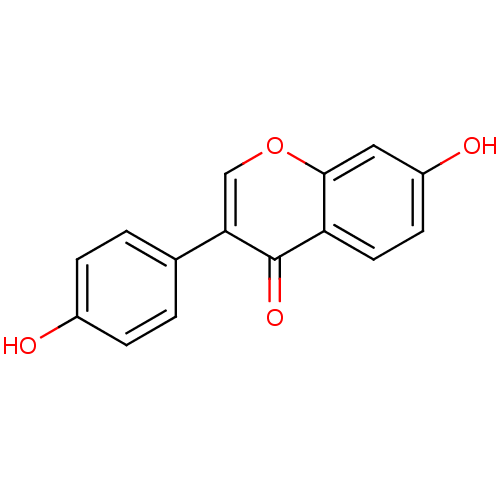 (7,4′-Dihydroxy-isoflavone (3a) | 7-hydroxy-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50561654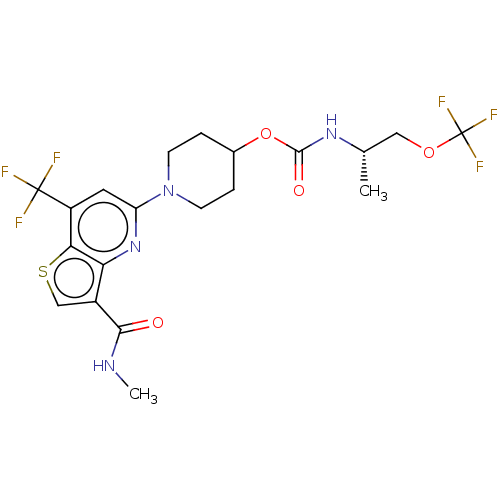 (CHEMBL4785914) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT1A1 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00120 BindingDB Entry DOI: 10.7270/Q2ZG6WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM25759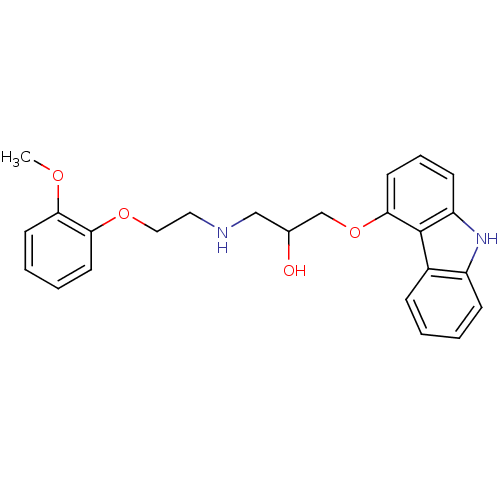 (CHEMBL723 | Carvedilol | Coreg | Dilatrend | Eucar...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM85507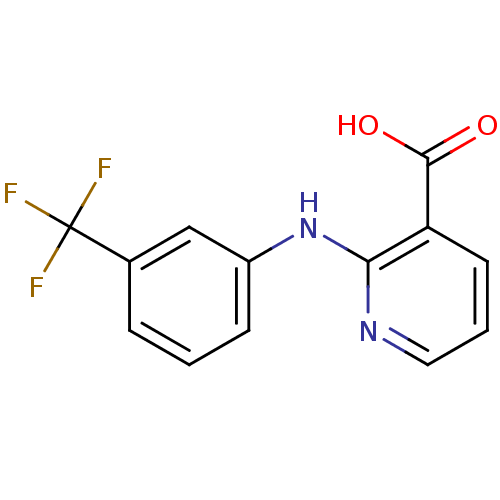 (CAS_4394-00-7 | NSC_4488 | Niflumic acid | Niflumi...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50001839 (CHEMBL428647 | PACLITAXEL | taxol) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50460861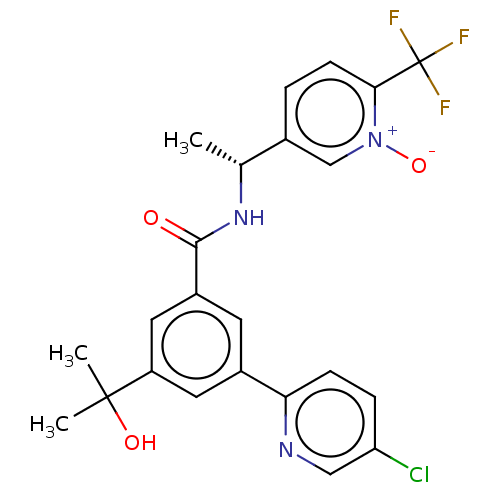 (CHEMBL4229237) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of UGT1A1 in human liver microsomes assessed as decrease in estradiol-3 glucuronidation | Bioorg Med Chem Lett 28: 1392-1396 (2018) Article DOI: 10.1016/j.bmcl.2018.02.039 BindingDB Entry DOI: 10.7270/Q25T3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50187243 (17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM21363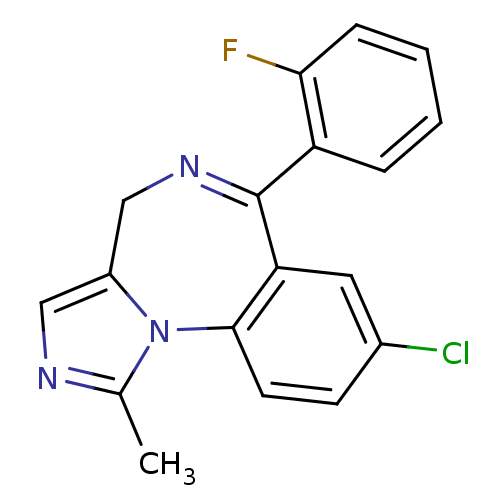 (12-chloro-9-(2-fluorophenyl)-3-methyl-2,4,8-triaza...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM25759 (CHEMBL723 | Carvedilol | Coreg | Dilatrend | Eucar...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM11021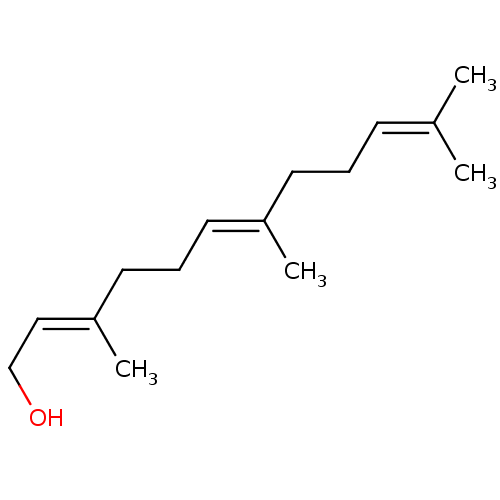 ((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol |...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM8885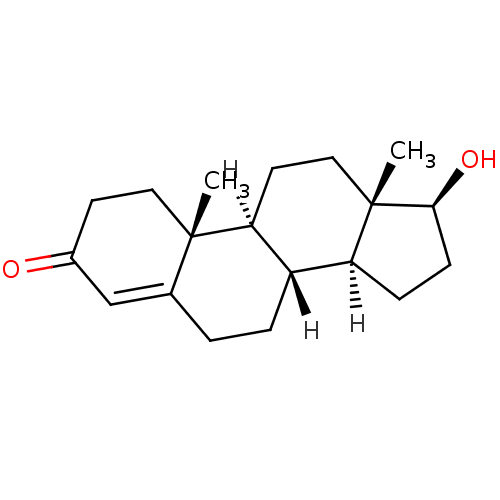 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50187243 (17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM234401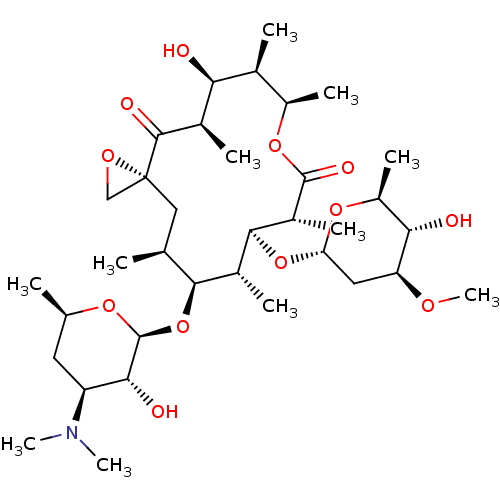 (Oleandomycin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM11021 ((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol |...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50508419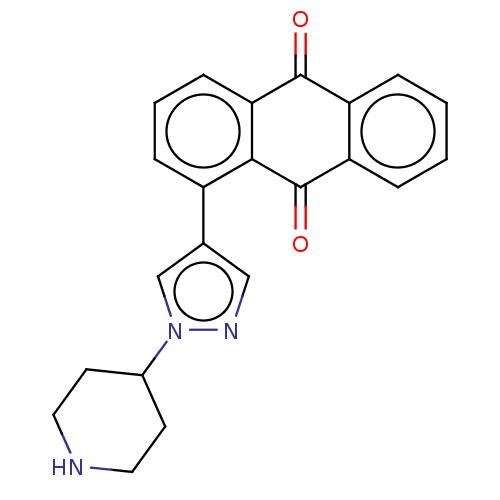 (CHEMBL4471264) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of UGT1A1 in human liver microsomes assessed as reduction in SN-38 glucuronidation by tandem mass spectrometry analysis | J Med Chem 62: 575-588 (2019) Article DOI: 10.1021/acs.jmedchem.8b01168 BindingDB Entry DOI: 10.7270/Q2QZ2F8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM85507 (CAS_4394-00-7 | NSC_4488 | Niflumic acid | Niflumi...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50241343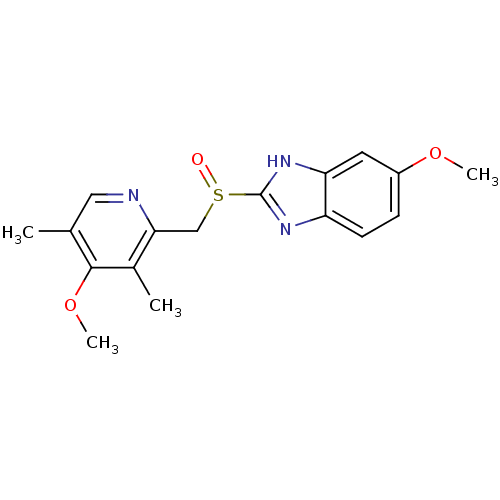 ((RS)-6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50022178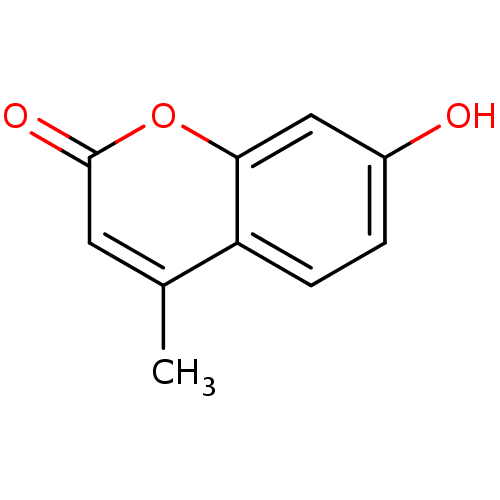 (4-Methyl-7-hydroxycoumarin | 4-methylumbelliferone...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50022178 (4-Methyl-7-hydroxycoumarin | 4-methylumbelliferone...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50062551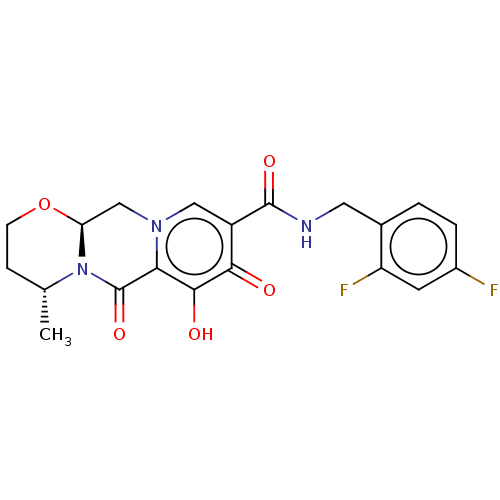 (CHEBI:76010 | Dolutegravir | GSK1349572 | S-349572) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in supersomes assessed as scopoletin glucuronidation by fluorescence analysis | Drug Metab Dispos 41: 353-61 (2013) Article DOI: 10.1124/dmd.112.048918 BindingDB Entry DOI: 10.7270/Q20003TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM50088507 (CHEBI:63804 | CHEMBL1908021) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method | Drug Metab Dispos 39: 322-9 (2011) Article DOI: 10.1124/dmd.110.035030 BindingDB Entry DOI: 10.7270/Q2PC343R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72 total ) | Next | Last >> |