

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50352164 (CHEMBL1824793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Batman University Curated by ChEMBL | Assay Description Competitive inhibition of human erythrocyte Glutathione reductase using GSSG substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 5398-402 (2011) Article DOI: 10.1016/j.bmcl.2011.07.002 BindingDB Entry DOI: 10.7270/Q2V69KKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM94597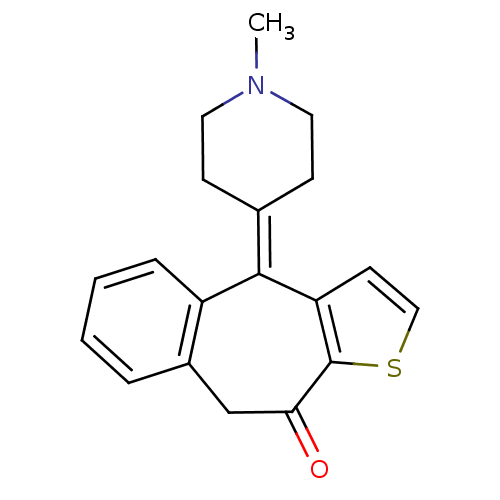 ((Z)-2-butenedioate;10-(1-methyl-4-piperidinylidene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 800 | -8.31 | 1.20E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Ataturk University | Assay Description GR activity was determined by the method of Carlberg and Mannervik [Carlberg et al., FL:Academic Press, 72:248-254] with a Shimadzu Spectrophotometer... | J Enzyme Inhib Med Chem 27: 18-23 (2012) Article DOI: 10.3109/14756366.2011.572879 BindingDB Entry DOI: 10.7270/Q20K27GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096018 (6-(3-METHYL-1,4-DIOXO-1,4-DIHYDRONAPHTHALEN-2-YL)H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase | J Med Chem 47: 5972-83 (2004) Article DOI: 10.1021/jm0497545 BindingDB Entry DOI: 10.7270/Q20R9NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50352161 (CHEMBL1824791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Batman University Curated by ChEMBL | Assay Description Non-Competitive inhibition of human erythrocyte Glutathione reductase using GSSG substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 5398-402 (2011) Article DOI: 10.1016/j.bmcl.2011.07.002 BindingDB Entry DOI: 10.7270/Q2V69KKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50352163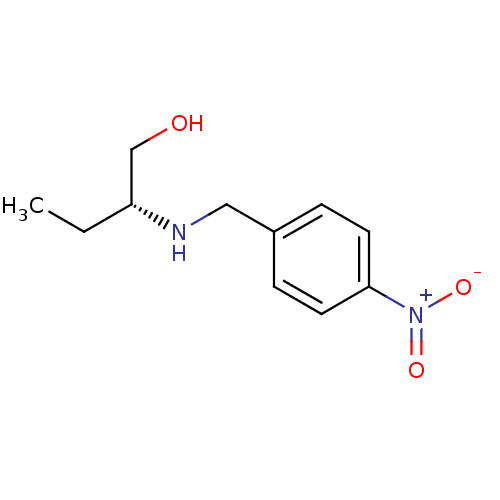 (CHEMBL1824792) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Batman University Curated by ChEMBL | Assay Description Competitive inhibition of human erythrocyte Glutathione reductase using GSSG substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 5398-402 (2011) Article DOI: 10.1016/j.bmcl.2011.07.002 BindingDB Entry DOI: 10.7270/Q2V69KKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50056998 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 6.10E+3 | -7.11 | 2.90E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Ataturk University | Assay Description GR activity was determined by the method of Carlberg and Mannervik [Carlberg et al., FL:Academic Press, 72:248-254] with a Shimadzu Spectrophotometer... | J Enzyme Inhib Med Chem 27: 18-23 (2012) Article DOI: 10.3109/14756366.2011.572879 BindingDB Entry DOI: 10.7270/Q20K27GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50422689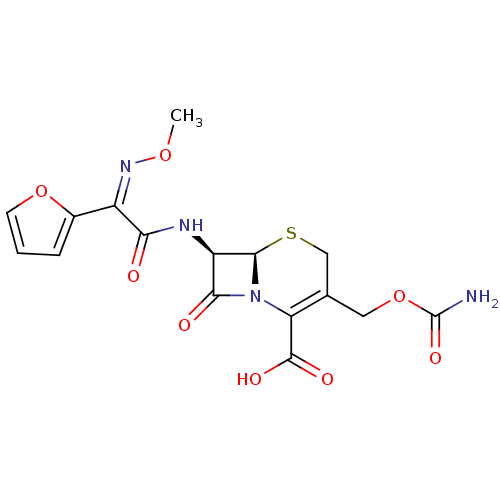 (640/359 | CEFUROXIME) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.04E+4 | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Ondokuz Mayis University | Assay Description Enzymatic activity was measured by Beutler's method with a Shimadzu Spectrophotometer UV-(1208), at 25°C. The assay system contained 100 mM Tris-... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50316592 ((2R(S),7R(S))-7-Hydroxybicyclo[2.2.1]heptan-2-yl n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM24778 (2-methyl-1,4-dihydronaphthalene-1,4-dione | 2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase (In presence of glutathione disulfide) | J Med Chem 47: 5972-83 (2004) Article DOI: 10.1021/jm0497545 BindingDB Entry DOI: 10.7270/Q20R9NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293668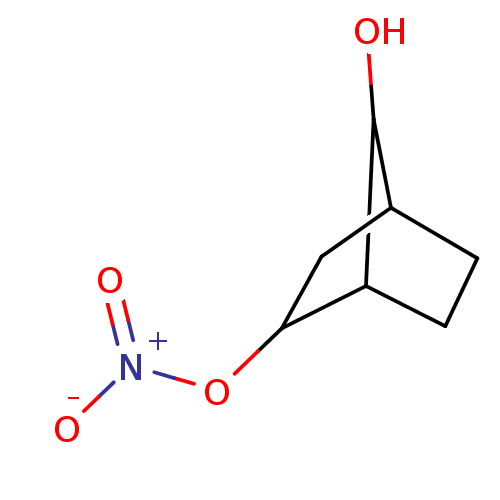 ((2S(R),7R(S))-7-Hydroxybicyclo[2.2.1]heptan-2-yl n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM24778 (2-methyl-1,4-dihydronaphthalene-1,4-dione | 2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase (In presence of NADPH) | J Med Chem 47: 5972-83 (2004) Article DOI: 10.1021/jm0497545 BindingDB Entry DOI: 10.7270/Q20R9NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50049707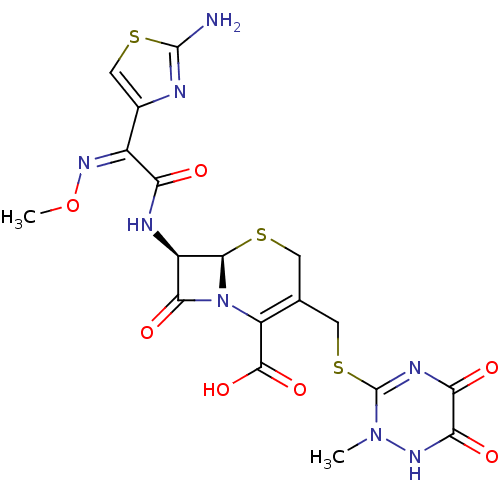 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.73E+4 | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Ondokuz Mayis University | Assay Description Enzymatic activity was measured by Beutler's method with a Shimadzu Spectrophotometer UV-(1208), at 25°C. The assay system contained 100 mM Tris-... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293667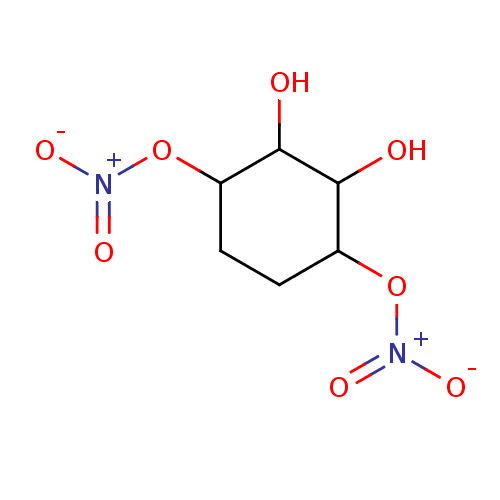 ((1R(S),2R(S),3S(R),4S(R))-2,3-Dihydroxycyclo-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50336640 ((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Batman University Curated by ChEMBL | Assay Description Competitive inhibition of human erythrocyte Glutathione reductase using GSSG substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 5398-402 (2011) Article DOI: 10.1016/j.bmcl.2011.07.002 BindingDB Entry DOI: 10.7270/Q2V69KKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293666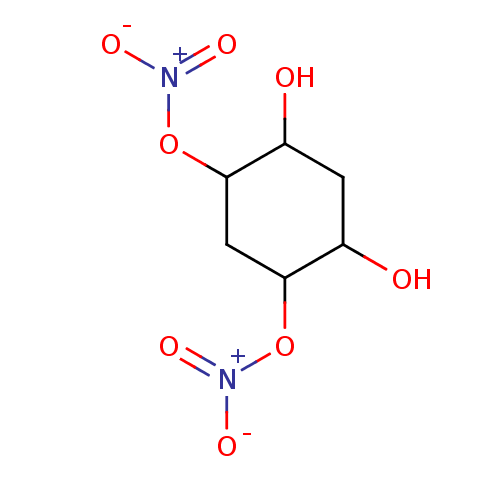 ((1S(R),3S(R),4S(R),6S(R))-4,6-Dihydroxycyclo-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293665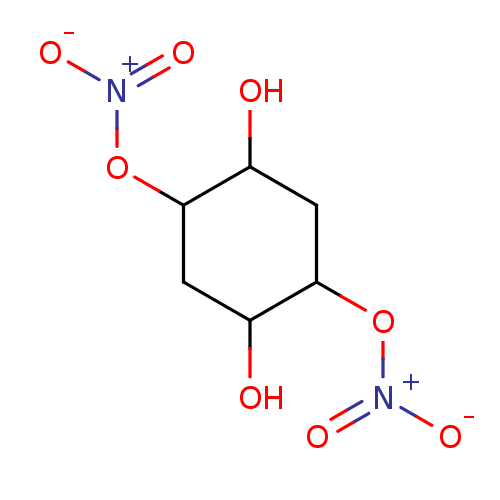 ((1R(S),2R(S),4R(S),5R(S))-2,5-Dihydroxycyclo-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293664 (9(R(S))-Hydroxy-1,2,3,4-tetrahydro-1,4-methano-nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293663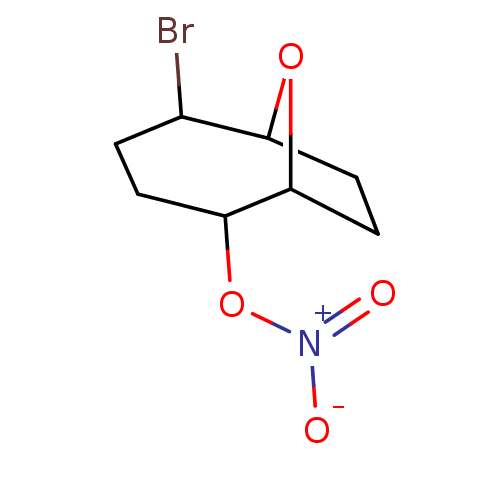 ((1S(R),2S(R),5R(S),6R(S))-5-Bromo-9-oxabicyclo[4.2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293661 (CHEMBL556256 | trans-(1S(R),2S(R))-2-Hydroxycycloo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM237182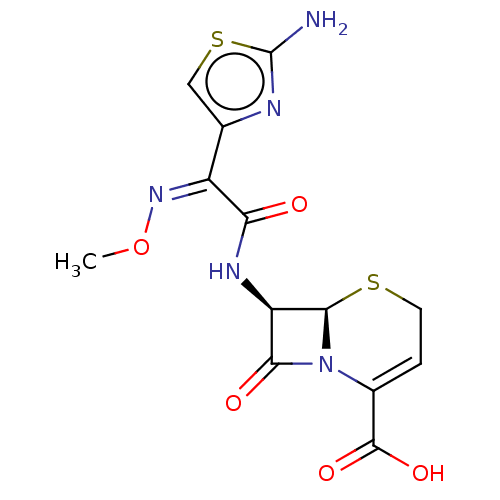 (Ceftizoxime) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | 2.28E+4 | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Ondokuz Mayis University | Assay Description Enzymatic activity was measured by Beutler's method with a Shimadzu Spectrophotometer UV-(1208), at 25°C. The assay system contained 100 mM Tris-... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293660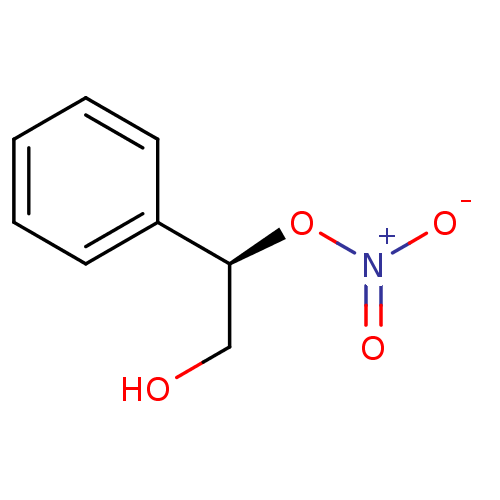 (CHEMBL563619 | trans-(R(S))-2-Hydroxy-1-phenylethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50390999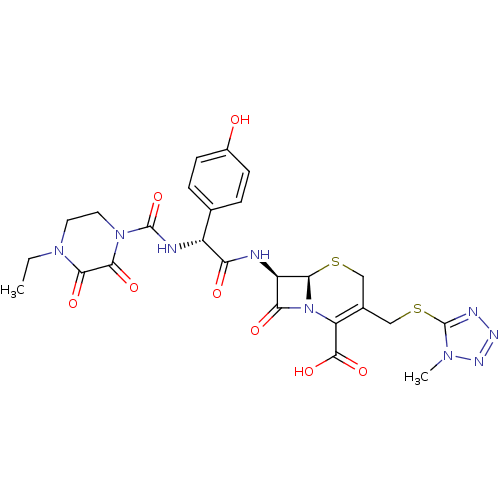 (CEFOPERAZONE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.87E+4 | n/a | 4.84E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Ondokuz Mayis University | Assay Description Enzymatic activity was measured by Beutler's method with a Shimadzu Spectrophotometer UV-(1208), at 25°C. The assay system contained 100 mM Tris-... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293658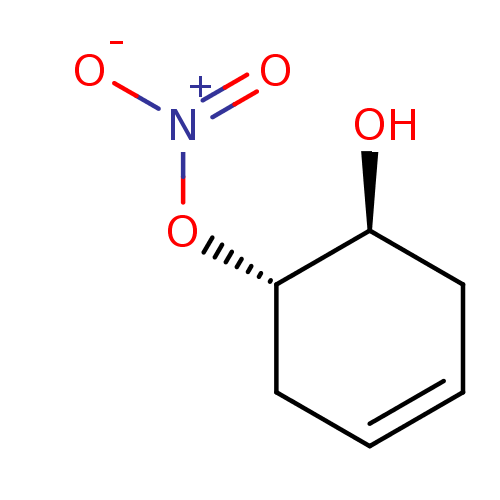 (CHEMBL555721 | trans-(1S(R),6S(R))-6-Hydroxycycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM17657 ((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Batman University Curated by ChEMBL | Assay Description Competitive inhibition of human erythrocyte Glutathione reductase using GSSG substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 5398-402 (2011) Article DOI: 10.1016/j.bmcl.2011.07.002 BindingDB Entry DOI: 10.7270/Q2V69KKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293659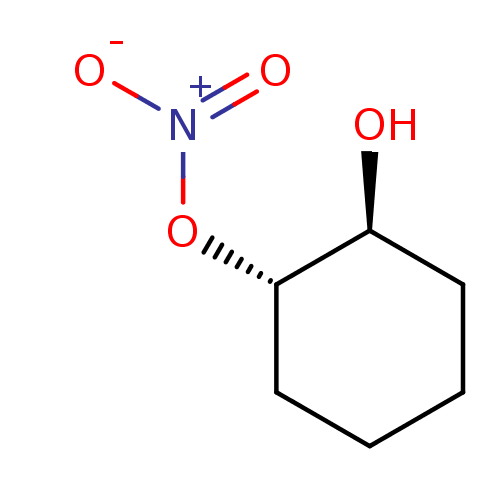 (CHEMBL562358 | trans-(1S(R),2S(R))-2-Hydroxycycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293662 (CHEMBL559615 | trans-(1S(R),8S(R),Z)-8-Hydroxycycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50352165 (LYSINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Batman University Curated by ChEMBL | Assay Description Competitive inhibition of human erythrocyte Glutathione reductase using GSSG substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 5398-402 (2011) Article DOI: 10.1016/j.bmcl.2011.07.002 BindingDB Entry DOI: 10.7270/Q2V69KKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50170723 (1,1'-Hexamethylene bis(5-(p-chlorophenyl)biguanide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Heidelberg Curated by ChEMBL | Assay Description Inhibitory constant against human glutathione reductase | J Med Chem 48: 4793-802 (2005) Article DOI: 10.1021/jm050027z BindingDB Entry DOI: 10.7270/Q2XK8GB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50352162 (NITROBENZENE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 2.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Batman University Curated by ChEMBL | Assay Description Competitive inhibition of human erythrocyte Glutathione reductase using GSSG substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 5398-402 (2011) Article DOI: 10.1016/j.bmcl.2011.07.002 BindingDB Entry DOI: 10.7270/Q2V69KKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM235694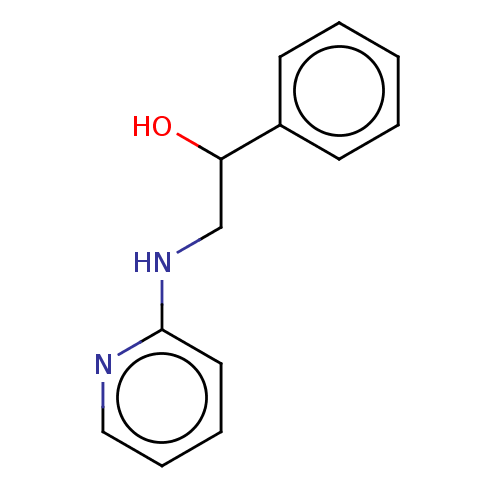 (Phenyramidol-HCl) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.34E+5 | -4.58 | 9.90E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Ataturk University | Assay Description GR activity was determined by the method of Carlberg and Mannervik [Carlberg et al., FL:Academic Press, 72:248-254] with a Shimadzu Spectrophotometer... | J Enzyme Inhib Med Chem 27: 18-23 (2012) Article DOI: 10.3109/14756366.2011.572879 BindingDB Entry DOI: 10.7270/Q20K27GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50091152 (CHEMBL106108 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Human Erythrocyte glutathione reductase (GR). | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50091149 ((4-Chloro-benzyl)-[3-(2-chloro-phenothiazin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Human Erythrocyte glutathione reductase (GR). | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50093207 ((E)-3-(3,4-Dihydroxy-phenyl)-N-[3-(4-{3-[(E)-3-(3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of human Glutathione reductase | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM233150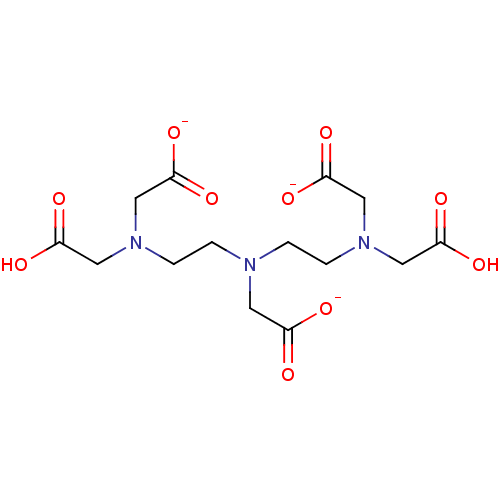 (Gadopentetic acid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 2.88E+7 | -2.10 | 1.38E+8 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Ataturk University | Assay Description GR activity was determined by the method of Carlberg and Mannervik [Carlberg et al., FL:Academic Press, 72:248-254] with a Shimadzu Spectrophotometer... | J Enzyme Inhib Med Chem 27: 18-23 (2012) Article DOI: 10.3109/14756366.2011.572879 BindingDB Entry DOI: 10.7270/Q20K27GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||