 Found 2829 hits of ic50 data for polymerid = 1024
Found 2829 hits of ic50 data for polymerid = 1024 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) transfected in human MCF7 cells |
J Med Chem 59: 5131-48 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01281
BindingDB Entry DOI: 10.7270/Q2TM7D2S |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50539776

(CHEMBL4639677)Show InChI InChI=1S/C22H18N2O3/c1-2-3-6-11-26-17-12-16(14-24-10-9-23-15-24)21-20(13-17)27-19-8-5-4-7-18(19)22(21)25/h4-5,7-10,12-13,15H,2,11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50539777

(CHEMBL4632445)Show InChI InChI=1S/C22H18N2O3/c1-2-3-4-11-26-17-6-8-19-21(13-17)27-20-12-16(5-7-18(20)22(19)25)14-24-10-9-23-15-24/h5-10,12-13,15H,2,11,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50337121
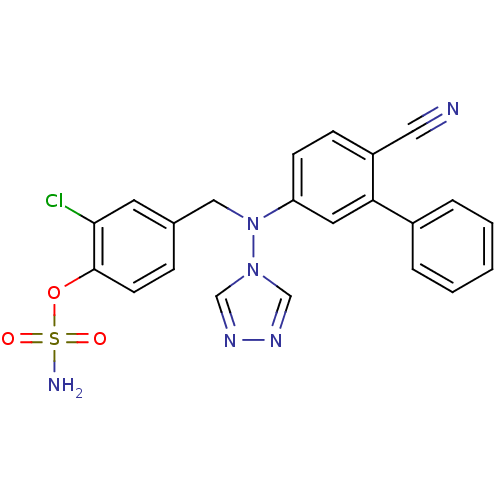
(2-chloro-4-(((6-cyanobiphenyl-3-yl)(4H-1,2,4-triaz...)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(C#N)c(c2)-c2ccccc2)n2cnnc2)cc1Cl Show InChI InChI=1S/C22H17ClN6O3S/c23-21-10-16(6-9-22(21)32-33(25,30)31)13-29(28-14-26-27-15-28)19-8-7-18(12-24)20(11-19)17-4-2-1-3-5-17/h1-11,14-15H,13H2,(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione after 1 hr by scintillation spectrometry |
ACS Med Chem Lett 2: 243-247 (2011)
Article DOI: 10.1021/ml100273k
BindingDB Entry DOI: 10.7270/Q2X067BN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50337123

(4-(((6-cyano-4'-methoxybiphenyl-3-yl)(4H-1,2,4-tri...)Show SMILES COc1ccc(cc1)-c1cc(ccc1C#N)N(Cc1ccc(OS(N)(=O)=O)cc1)n1cnnc1 Show InChI InChI=1S/C23H20N6O4S/c1-32-21-10-5-18(6-11-21)23-12-20(7-4-19(23)13-24)29(28-15-26-27-16-28)14-17-2-8-22(9-3-17)33-34(25,30)31/h2-12,15-16H,14H2,1H3,(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione after 1 hr by scintillation spectrometry |
ACS Med Chem Lett 2: 243-247 (2011)
Article DOI: 10.1021/ml100273k
BindingDB Entry DOI: 10.7270/Q2X067BN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50337122
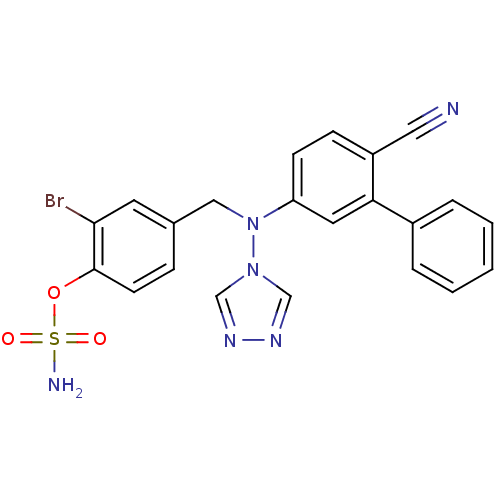
(2-bromo-4-(((6-cyanobiphenyl-3-yl)(4H-1,2,4-triazo...)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(C#N)c(c2)-c2ccccc2)n2cnnc2)cc1Br Show InChI InChI=1S/C22H17BrN6O3S/c23-21-10-16(6-9-22(21)32-33(25,30)31)13-29(28-14-26-27-15-28)19-8-7-18(12-24)20(11-19)17-4-2-1-3-5-17/h1-11,14-15H,13H2,(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione after 1 hr by scintillation spectrometry |
ACS Med Chem Lett 2: 243-247 (2011)
Article DOI: 10.1021/ml100273k
BindingDB Entry DOI: 10.7270/Q2X067BN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Aromatase inhibitor potency as iron-binding-related type II difference spectrum |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50337118
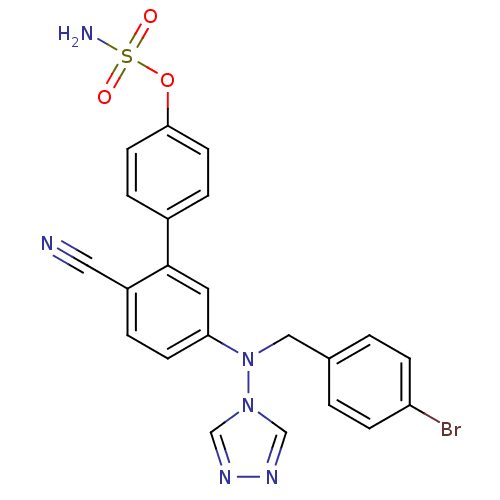
(5'-((4-bromobenzyl)(4H-1,2,4-triazol-4-yl)amino)-2...)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cc(ccc1C#N)N(Cc1ccc(Br)cc1)n1cnnc1 Show InChI InChI=1S/C22H17BrN6O3S/c23-19-6-1-16(2-7-19)13-29(28-14-26-27-15-28)20-8-3-18(12-24)22(11-20)17-4-9-21(10-5-17)32-33(25,30)31/h1-11,14-15H,13H2,(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione after 1 hr by scintillation spectrometry |
ACS Med Chem Lett 2: 243-247 (2011)
Article DOI: 10.1021/ml100273k
BindingDB Entry DOI: 10.7270/Q2X067BN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592781
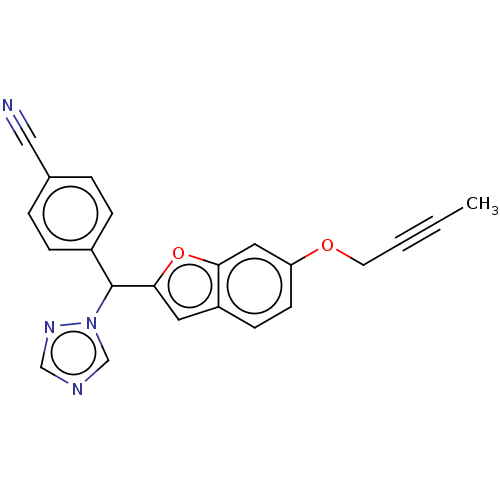
(CHEMBL5203413)Show SMILES CC#CCOc1ccc2cc(oc2c1)C(c1ccc(cc1)C#N)n1cncn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50337117

(5'-(benzyl(4H-1,2,4-triazol-4-yl)amino)-2'-cyanobi...)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cc(ccc1C#N)N(Cc1ccccc1)n1cnnc1 Show InChI InChI=1S/C22H18N6O3S/c23-13-19-6-9-20(12-22(19)18-7-10-21(11-8-18)31-32(24,29)30)28(27-15-25-26-16-27)14-17-4-2-1-3-5-17/h1-12,15-16H,14H2,(H2,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione after 1 hr by scintillation spectrometry |
ACS Med Chem Lett 2: 243-247 (2011)
Article DOI: 10.1021/ml100273k
BindingDB Entry DOI: 10.7270/Q2X067BN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307918

(2-(5-((1H-1,2,4-triazol-1-yl)methyl)-3'-chloro-4'-...)Show SMILES CC(C)(C#N)c1cc(Cn2cncn2)cc(c1)-c1ccc(O)c(Cl)c1 Show InChI InChI=1S/C19H17ClN4O/c1-19(2,10-21)16-6-13(9-24-12-22-11-23-24)5-15(7-16)14-3-4-18(25)17(20)8-14/h3-8,11-12,25H,9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123025
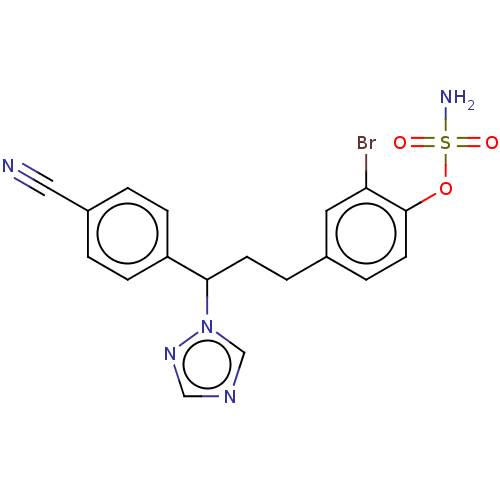
(CHEMBL3623231)Show SMILES NS(=O)(=O)Oc1ccc(CCC(c2ccc(cc2)C#N)n2cncn2)cc1Br Show InChI InChI=1S/C18H16BrN5O3S/c19-16-9-13(4-8-18(16)27-28(21,25)26)3-7-17(24-12-22-11-23-24)15-5-1-14(10-20)2-6-15/h1-2,4-6,8-9,11-12,17H,3,7H2,(H2,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10007
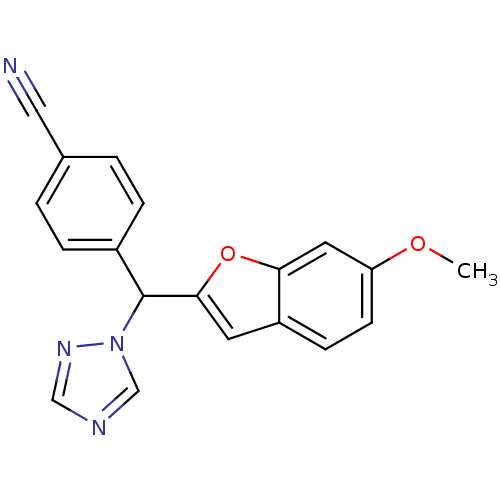
(4-[(6-methoxy-1-benzofuran-2-yl)(1H-1,2,4-triazol-...)Show InChI InChI=1S/C19H14N4O2/c1-24-16-7-6-15-8-18(25-17(15)9-16)19(23-12-21-11-22-23)14-4-2-13(10-20)3-5-14/h2-9,11-12,19H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10016

(4-{[(4-bromophenyl)methyl](4H-1,2,4-triazol-4-yl)a...)Show InChI InChI=1S/C16H12BrN5/c17-15-5-1-14(2-6-15)10-22(21-11-19-20-12-21)16-7-3-13(9-18)4-8-16/h1-8,11-12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human placental microsome |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525257

(CHEMBL4520995)Show SMILES [O-][N+](=O)c1ccc(cc1)N(Cc1ccc(Br)cc1)n1cnnc1 Show InChI InChI=1S/C15H12BrN5O2/c16-13-3-1-12(2-4-13)9-20(19-10-17-18-11-19)14-5-7-15(8-6-14)21(22)23/h1-8,10-11H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate by liquid scintillation counting method |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307884
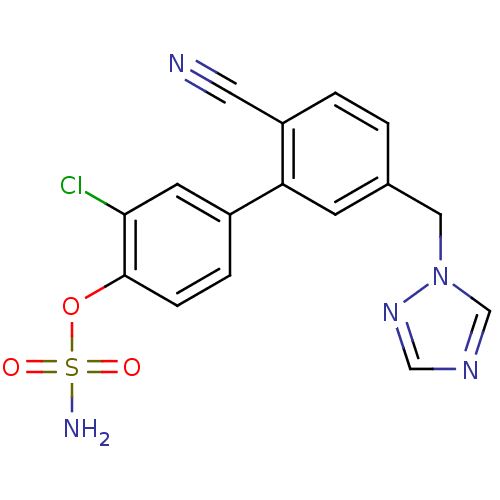
(3-CHLORO-2'-CYANO-5'-(1H-1,2,4-TRIAZOL-1-YLMETHYL)...)Show SMILES NS(=O)(=O)Oc1ccc(cc1Cl)-c1cc(Cn2cncn2)ccc1C#N Show InChI InChI=1S/C16H12ClN5O3S/c17-15-6-12(3-4-16(15)25-26(19,23)24)14-5-11(1-2-13(14)7-18)8-22-10-20-9-21-22/h1-6,9-10H,8H2,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) expressed in JEG-3 cells |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10004

(1-[(4-fluorophenyl)(6-methoxy-1-benzofuran-2-yl)me...)Show InChI InChI=1S/C18H14FN3O2/c1-23-15-7-4-13-8-17(24-16(13)9-15)18(22-11-20-10-21-22)12-2-5-14(19)6-3-12/h2-11,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307901
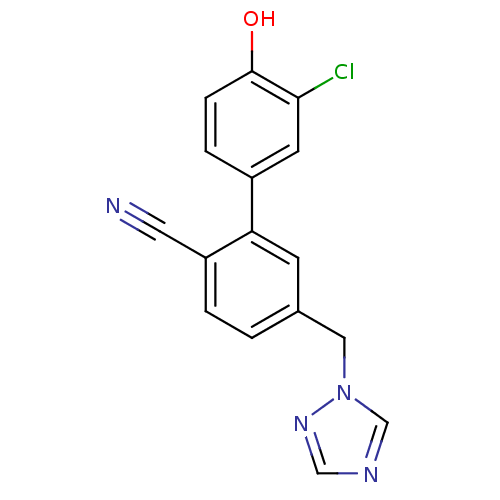
(5-((1H-1,2,4-triazol-1-yl)methyl)-3'-chloro-4'-hyd...)Show InChI InChI=1S/C16H11ClN4O/c17-15-6-12(3-4-16(15)22)14-5-11(1-2-13(14)7-18)8-21-10-19-9-20-21/h1-6,9-10,22H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) expressed in JEG-3 cells |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50337120
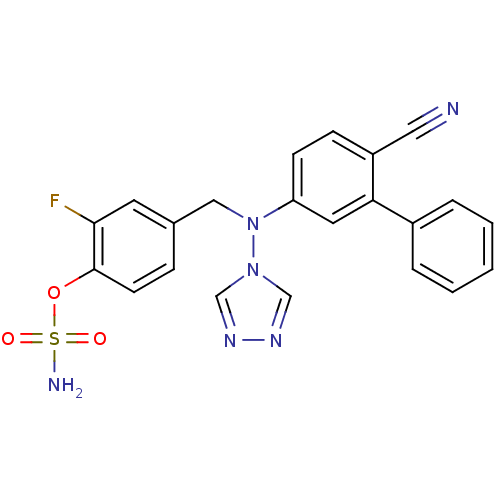
(4-(((6-cyanobiphenyl-3-yl)(4H-1,2,4-triazol-4-yl)a...)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(C#N)c(c2)-c2ccccc2)n2cnnc2)cc1F Show InChI InChI=1S/C22H17FN6O3S/c23-21-10-16(6-9-22(21)32-33(25,30)31)13-29(28-14-26-27-15-28)19-8-7-18(12-24)20(11-19)17-4-2-1-3-5-17/h1-11,14-15H,13H2,(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione after 1 hr by scintillation spectrometry |
ACS Med Chem Lett 2: 243-247 (2011)
Article DOI: 10.1021/ml100273k
BindingDB Entry DOI: 10.7270/Q2X067BN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307901

(5-((1H-1,2,4-triazol-1-yl)methyl)-3'-chloro-4'-hyd...)Show InChI InChI=1S/C16H11ClN4O/c17-15-6-12(3-4-16(15)22)14-5-11(1-2-13(14)7-18)8-21-10-19-9-20-21/h1-6,9-10,22H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM24341
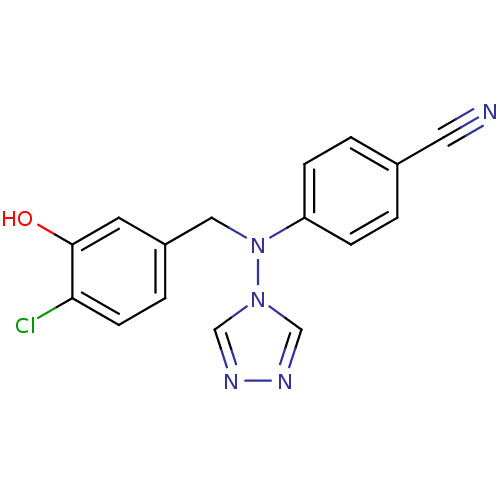
(4-{[(4-chloro-3-hydroxyphenyl)methyl](4H-1,2,4-tri...)Show InChI InChI=1S/C16H12ClN5O/c17-15-6-3-13(7-16(15)23)9-22(21-10-19-20-11-21)14-4-1-12(8-18)2-5-14/h1-7,10-11,23H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath
| Assay Description
The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... |
J Med Chem 50: 3540-60 (2007)
Article DOI: 10.1021/jm061462b
BindingDB Entry DOI: 10.7270/Q2348HP4 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123026
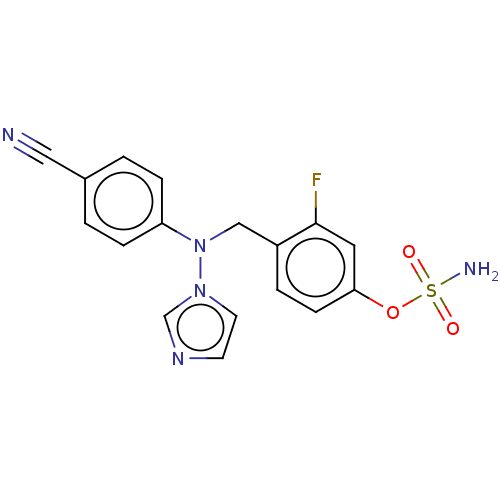
(CHEMBL3623232)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2ccnc2)c(F)c1 Show InChI InChI=1S/C17H14FN5O3S/c18-17-9-16(26-27(20,24)25)6-3-14(17)11-23(22-8-7-21-12-22)15-4-1-13(10-19)2-5-15/h1-9,12H,11H2,(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG3 cells using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 1 hr by scintillati... |
J Med Chem 59: 5131-48 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01281
BindingDB Entry DOI: 10.7270/Q2TM7D2S |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123026

(CHEMBL3623232)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2ccnc2)c(F)c1 Show InChI InChI=1S/C17H14FN5O3S/c18-17-9-16(26-27(20,24)25)6-3-14(17)11-23(22-8-7-21-12-22)15-4-1-13(10-19)2-5-15/h1-9,12H,11H2,(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525260
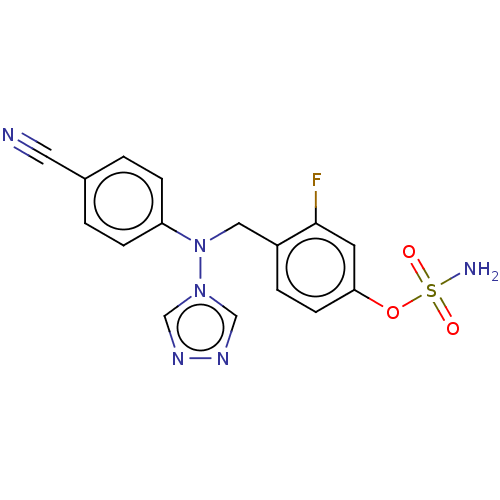
(CHEMBL4465348)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2cnnc2)c(F)c1 Show InChI InChI=1S/C16H13FN6O3S/c17-16-7-15(26-27(19,24)25)6-3-13(16)9-23(22-10-20-21-11-22)14-4-1-12(8-18)2-5-14/h1-7,10-11H,9H2,(H2,19,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307900
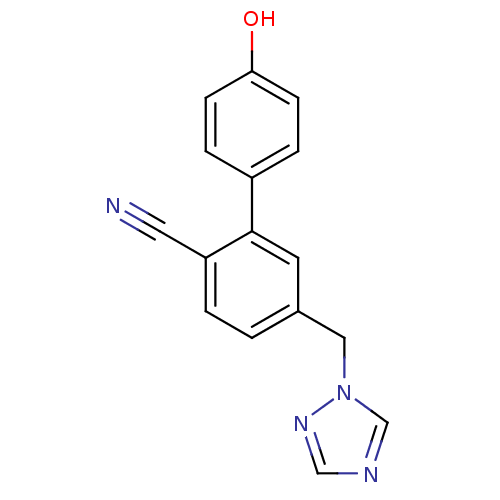
(5-((1H-1,2,4-triazol-1-yl)methyl)-4'-hydroxybiphen...)Show InChI InChI=1S/C16H12N4O/c17-8-14-2-1-12(9-20-11-18-10-19-20)7-16(14)13-3-5-15(21)6-4-13/h1-7,10-11,21H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307899

(5-((1H-1,2,4-triazol-1-yl)methyl)biphenyl-2-carbon...)Show InChI InChI=1S/C16H12N4/c17-9-15-7-6-13(10-20-12-18-11-19-20)8-16(15)14-4-2-1-3-5-14/h1-8,11-12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50337119

(4-(((6-cyanobiphenyl-3-yl)(4H-1,2,4-triazol-4-yl)a...)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(C#N)c(c2)-c2ccccc2)n2cnnc2)cc1 Show InChI InChI=1S/C22H18N6O3S/c23-13-19-8-9-20(12-22(19)18-4-2-1-3-5-18)28(27-15-25-26-16-27)14-17-6-10-21(11-7-17)31-32(24,29)30/h1-12,15-16H,14H2,(H2,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione after 1 hr by scintillation spectrometry |
ACS Med Chem Lett 2: 243-247 (2011)
Article DOI: 10.1021/ml100273k
BindingDB Entry DOI: 10.7270/Q2X067BN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307902
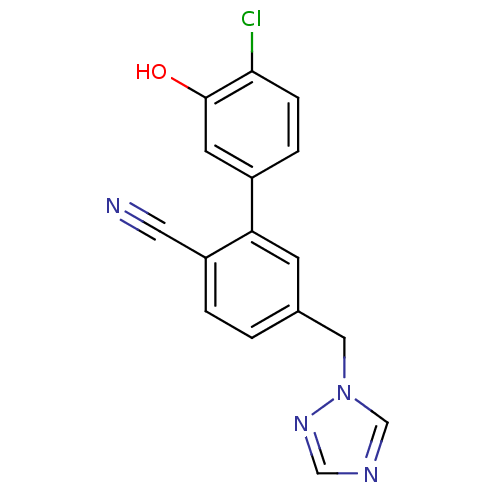
(5-((1H-1,2,4-triazol-1-yl)methyl)-4'-chloro-3'-hyd...)Show InChI InChI=1S/C16H11ClN4O/c17-15-4-3-12(6-16(15)22)14-5-11(1-2-13(14)7-18)8-21-10-19-9-20-21/h1-6,9-10,22H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307919
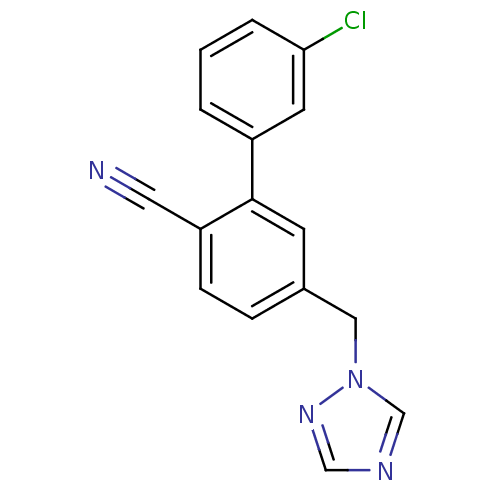
(5-((1H-1,2,4-triazol-1-yl)methyl)-3'-chlorobipheny...)Show InChI InChI=1S/C16H11ClN4/c17-15-3-1-2-13(7-15)16-6-12(4-5-14(16)8-18)9-21-11-19-10-20-21/h1-7,10-11H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121079

(CHEMBL3622064)Show SMILES NS(=O)(=O)Oc1ccc2cc(CN(c3ccc(cc3)C#N)n3cnnc3)ccc2c1Br Show InChI InChI=1S/C20H15BrN6O3S/c21-20-18-7-3-15(9-16(18)4-8-19(20)30-31(23,28)29)11-27(26-12-24-25-13-26)17-5-1-14(10-22)2-6-17/h1-9,12-13H,11H2,(H2,23,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121079

(CHEMBL3622064)Show SMILES NS(=O)(=O)Oc1ccc2cc(CN(c3ccc(cc3)C#N)n3cnnc3)ccc2c1Br Show InChI InChI=1S/C20H15BrN6O3S/c21-20-18-7-3-15(9-16(18)4-8-19(20)30-31(23,28)29)11-27(26-12-24-25-13-26)17-5-1-14(10-22)2-6-17/h1-9,12-13H,11H2,(H2,23,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121079

(CHEMBL3622064)Show SMILES NS(=O)(=O)Oc1ccc2cc(CN(c3ccc(cc3)C#N)n3cnnc3)ccc2c1Br Show InChI InChI=1S/C20H15BrN6O3S/c21-20-18-7-3-15(9-16(18)4-8-19(20)30-31(23,28)29)11-27(26-12-24-25-13-26)17-5-1-14(10-22)2-6-17/h1-9,12-13H,11H2,(H2,23,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) expressed in JEG-3 cells |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121105
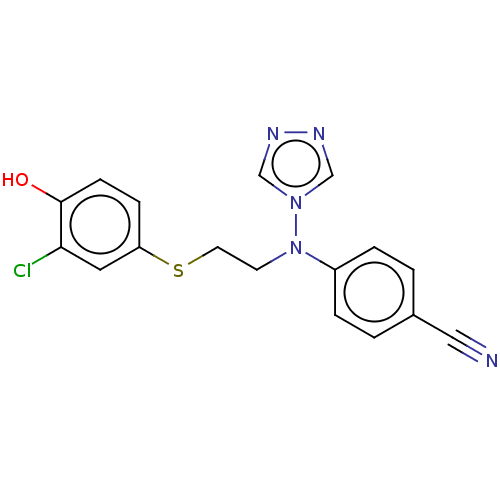
(CHEMBL3622055)Show InChI InChI=1S/C17H14ClN5OS/c18-16-9-15(5-6-17(16)24)25-8-7-23(22-11-20-21-12-22)14-3-1-13(10-19)2-4-14/h1-6,9,11-12,24H,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) expressed in JEG-3 cells |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121105

(CHEMBL3622055)Show InChI InChI=1S/C17H14ClN5OS/c18-16-9-15(5-6-17(16)24)25-8-7-23(22-11-20-21-12-22)14-3-1-13(10-19)2-4-14/h1-6,9,11-12,24H,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121095
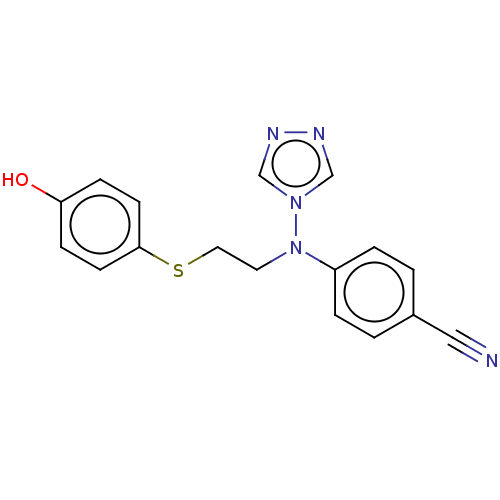
(CHEMBL3622054)Show InChI InChI=1S/C17H15N5OS/c18-11-14-1-3-15(4-2-14)22(21-12-19-20-13-21)9-10-24-17-7-5-16(23)6-8-17/h1-8,12-13,23H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) expressed in JEG-3 cells |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307881
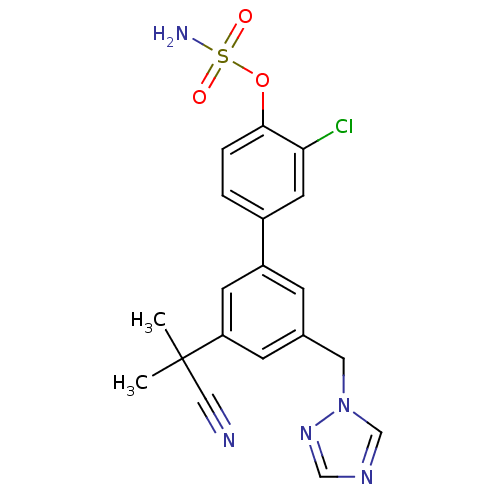
(3'-((1H-1,2,4-triazol-1-yl)methyl)-3-chloro-5'-(2-...)Show SMILES CC(C)(C#N)c1cc(Cn2cncn2)cc(c1)-c1ccc(OS(N)(=O)=O)c(Cl)c1 Show InChI InChI=1S/C19H18ClN5O3S/c1-19(2,10-21)16-6-13(9-25-12-23-11-24-25)5-15(7-16)14-3-4-18(17(20)8-14)28-29(22,26)27/h3-8,11-12H,9H2,1-2H3,(H2,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM24328
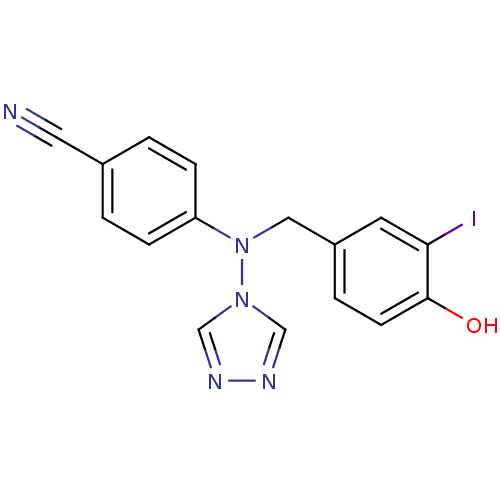
(4-{[(4-hydroxy-3-iodophenyl)methyl](4H-1,2,4-triaz...)Show InChI InChI=1S/C16H12IN5O/c17-15-7-13(3-6-16(15)23)9-22(21-10-19-20-11-21)14-4-1-12(8-18)2-5-14/h1-7,10-11,23H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath
| Assay Description
The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... |
J Med Chem 50: 3540-60 (2007)
Article DOI: 10.1021/jm061462b
BindingDB Entry DOI: 10.7270/Q2348HP4 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307882

(5'-((1H-1,2,4-triazol-1-yl)methyl)-4-chloro-2'-cya...)Show SMILES NS(=O)(=O)Oc1cc(ccc1Cl)-c1cc(Cn2cncn2)ccc1C#N Show InChI InChI=1S/C16H12ClN5O3S/c17-15-4-3-12(6-16(15)25-26(19,23)24)14-5-11(1-2-13(14)7-18)8-22-10-20-9-21-22/h1-6,9-10H,8H2,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592778

(CHEMBL5175340) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10016

(4-{[(4-bromophenyl)methyl](4H-1,2,4-triazol-4-yl)a...)Show InChI InChI=1S/C16H12BrN5/c17-15-5-1-14(2-6-15)10-22(21-11-19-20-12-21)16-7-3-13(9-18)4-8-16/h1-8,11-12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate by liquid scintillation counting method |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | CHEMBL5271052

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM24344
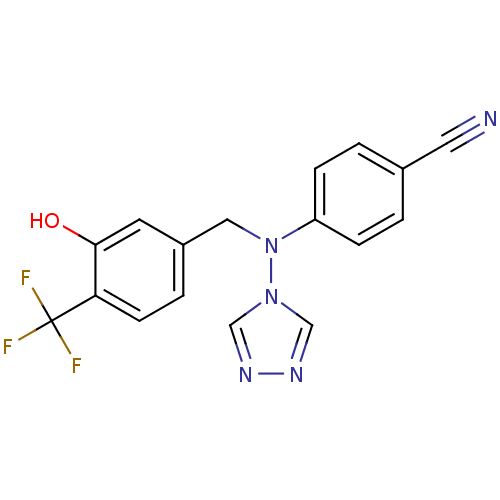
(4-({[3-hydroxy-4-(trifluoromethyl)phenyl]methyl}(4...)Show SMILES Oc1cc(CN(c2ccc(cc2)C#N)n2cnnc2)ccc1C(F)(F)F Show InChI InChI=1S/C17H12F3N5O/c18-17(19,20)15-6-3-13(7-16(15)26)9-25(24-10-22-23-11-24)14-4-1-12(8-21)2-5-14/h1-7,10-11,26H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
| Assay Description
The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... |
J Med Chem 50: 3540-60 (2007)
Article DOI: 10.1021/jm061462b
BindingDB Entry DOI: 10.7270/Q2348HP4 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121073

(CHEMBL3622056)Show SMILES NS(=O)(=O)Oc1ccc(SCCN(c2ccc(cc2)C#N)n2cnnc2)cc1 Show InChI InChI=1S/C17H16N6O3S2/c18-11-14-1-3-15(4-2-14)23(22-12-20-21-13-22)9-10-27-17-7-5-16(6-8-17)26-28(19,24)25/h1-8,12-13H,9-10H2,(H2,19,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) expressed in JEG-3 cells |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50460601

(CHEMBL4227385)Show InChI InChI=1S/C13H9N3OS/c17-11-5-2-1-4-9(11)10-8-18-13(16-10)12-14-6-3-7-15-12/h1-8,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul Medipol University
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Bioorg Med Chem 26: 1986-1995 (2018)
Article DOI: 10.1016/j.bmc.2018.02.048
BindingDB Entry DOI: 10.7270/Q20867Z3 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121074

(CHEMBL3622057)Show SMILES NS(=O)(=O)Oc1ccc(SCCN(c2ccc(cc2)C#N)n2cnnc2)cc1Cl Show InChI InChI=1S/C17H15ClN6O3S2/c18-16-9-15(5-6-17(16)27-29(20,25)26)28-8-7-24(23-11-21-22-12-23)14-3-1-13(10-19)2-4-14/h1-6,9,11-12H,7-8H2,(H2,20,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) expressed in JEG-3 cells |
J Med Chem 58: 7634-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00386
BindingDB Entry DOI: 10.7270/Q2474CP9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50592775

(CHEMBL5184004) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10001
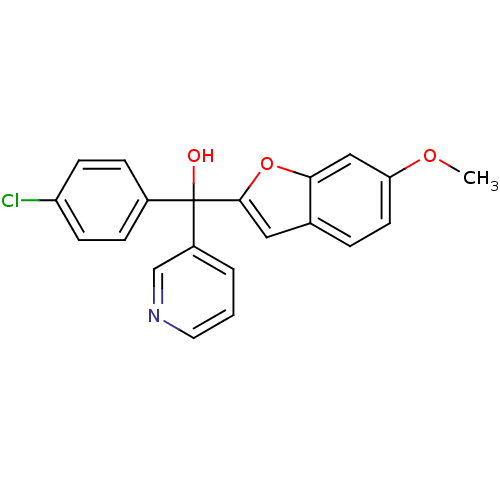
((4-chlorophenyl)(6-methoxy-1-benzofuran-2-yl)pyrid...)Show SMILES COc1ccc2cc(oc2c1)C(O)(c1ccc(Cl)cc1)c1cccnc1 Show InChI InChI=1S/C21H16ClNO3/c1-25-18-9-4-14-11-20(26-19(14)12-18)21(24,16-3-2-10-23-13-16)15-5-7-17(22)8-6-15/h2-13,24H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50337124
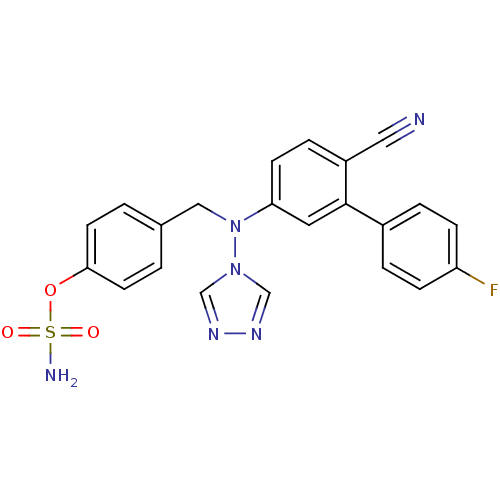
(4-(((6-cyano-4'-fluorobiphenyl-3-yl)(4H-1,2,4-tria...)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(C#N)c(c2)-c2ccc(F)cc2)n2cnnc2)cc1 Show InChI InChI=1S/C22H17FN6O3S/c23-19-6-3-17(4-7-19)22-11-20(8-5-18(22)12-24)29(28-14-26-27-15-28)13-16-1-9-21(10-2-16)32-33(25,30)31/h1-11,14-15H,13H2,(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione after 1 hr by scintillation spectrometry |
ACS Med Chem Lett 2: 243-247 (2011)
Article DOI: 10.1021/ml100273k
BindingDB Entry DOI: 10.7270/Q2X067BN |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10005
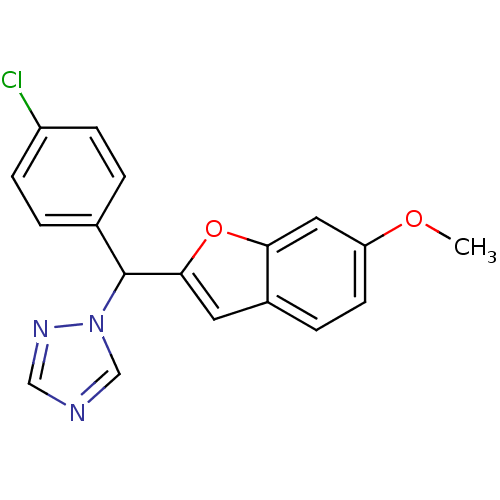
(1-[(4-chlorophenyl)(6-methoxy-1-benzofuran-2-yl)me...)Show InChI InChI=1S/C18H14ClN3O2/c1-23-15-7-4-13-8-17(24-16(13)9-15)18(22-11-20-10-21-22)12-2-5-14(19)6-3-12/h2-11,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50307884

(3-CHLORO-2'-CYANO-5'-(1H-1,2,4-TRIAZOL-1-YLMETHYL)...)Show SMILES NS(=O)(=O)Oc1ccc(cc1Cl)-c1cc(Cn2cncn2)ccc1C#N Show InChI InChI=1S/C16H12ClN5O3S/c17-15-6-12(3-4-16(15)25-26(19,23)24)14-5-11(1-2-13(14)7-18)8-22-10-20-9-21-22/h1-6,9-10H,8H2,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of aromatse in human JEG3 cells by scintillation spectrometry |
J Med Chem 53: 2155-70 (2010)
Article DOI: 10.1021/jm901705h
BindingDB Entry DOI: 10.7270/Q2959JGF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data