

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Bacillus anthracis) | BDBM50421214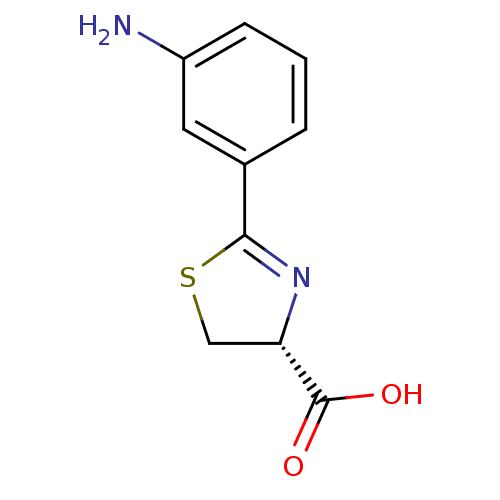 (CHEMBL2087633) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis MBL Bla2 using nitrocefin as substrate preincubated for 20 mins by UV-spectrophotometric analysis | Bioorg Med Chem Lett 22: 6229-32 (2012) Article DOI: 10.1016/j.bmcl.2012.08.012 BindingDB Entry DOI: 10.7270/Q2CF9RCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus anthracis) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis MBL Bla2 using nitrocefin as substrate preincubated for 20 mins by UV-spectrophotometric analysis | Bioorg Med Chem Lett 22: 6229-32 (2012) Article DOI: 10.1016/j.bmcl.2012.08.012 BindingDB Entry DOI: 10.7270/Q2CF9RCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Bacillus anthracis) | BDBM50335803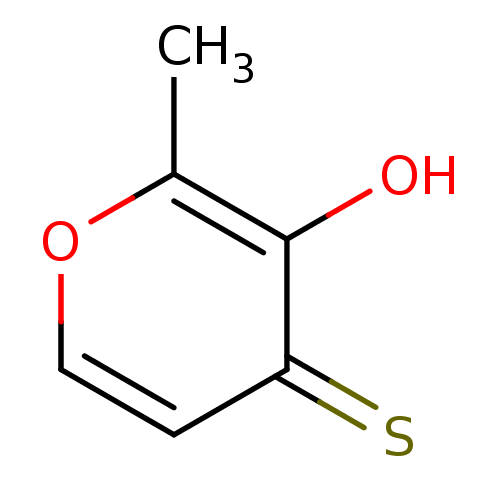 (3-hydroxy-2-methyl-4H-pyran-4-thione | CHEMBL16506...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Baylor University | Assay Description Progress curves for slow-binding inhibition analysis were obtained by adding nitrocefin (final concentration 36 μM) to a solution containing enz... | J Enzyme Inhib Med Chem 28: 137-42 (2013) Article DOI: 10.3109/14756366.2011.640632 BindingDB Entry DOI: 10.7270/Q26T0KHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||