

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50235322 (CHEMBL4066593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat cortex AchE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 128: 332-345 (2017) Article DOI: 10.1016/j.ejmech.2016.10.060 BindingDB Entry DOI: 10.7270/Q2HM5BQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9054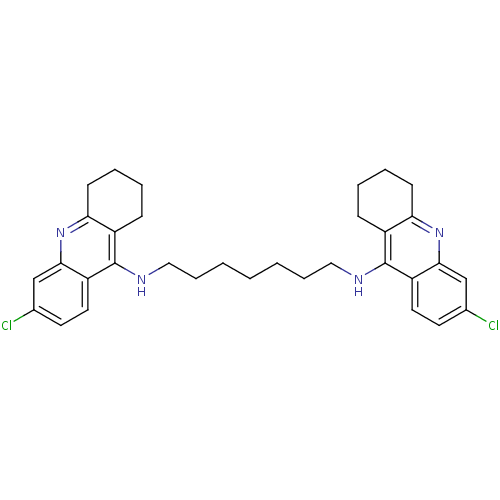 (6-chloro-N-{7-[(6-chloro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50235323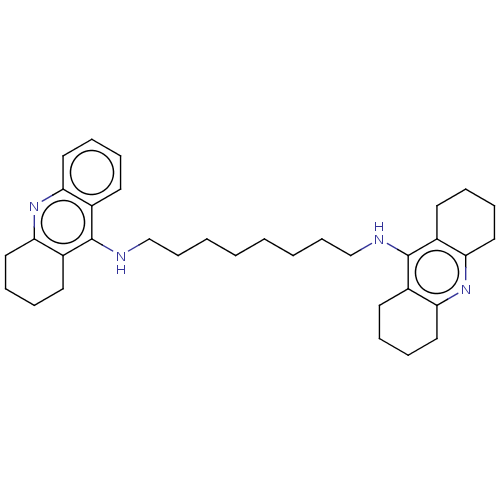 (CHEMBL4080023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat cortex AchE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 128: 332-345 (2017) Article DOI: 10.1016/j.ejmech.2016.10.060 BindingDB Entry DOI: 10.7270/Q2HM5BQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50235324 (CHEMBL4104258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat cortex AchE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 128: 332-345 (2017) Article DOI: 10.1016/j.ejmech.2016.10.060 BindingDB Entry DOI: 10.7270/Q2HM5BQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9055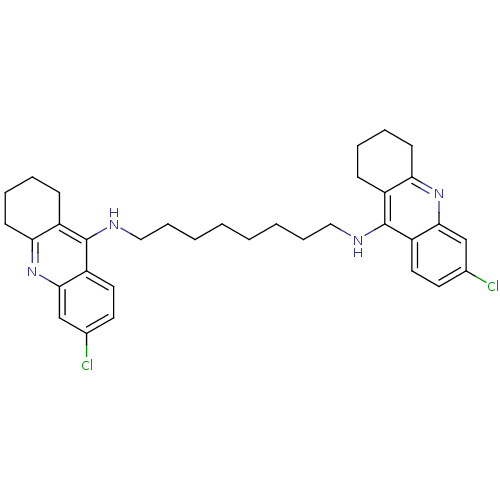 (6-chloro-N-{8-[(6-chloro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50037158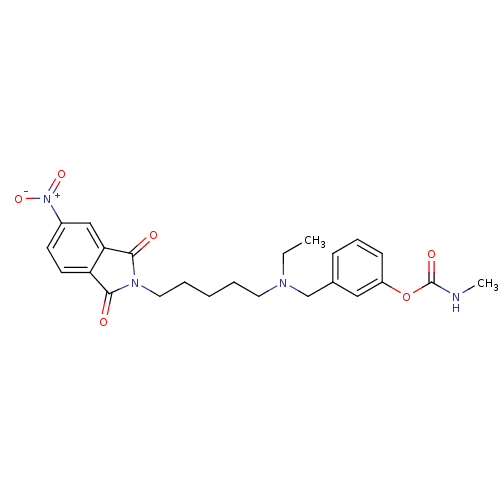 (CHEMBL106739 | Methyl-carbamic acid 3-({ethyl-[5-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition concentration against rat acetylcholinesterase | J Med Chem 37: 3141-53 (1994) BindingDB Entry DOI: 10.7270/Q2JS9PHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128602 BindingDB Entry DOI: 10.7270/Q2HM5DF5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9047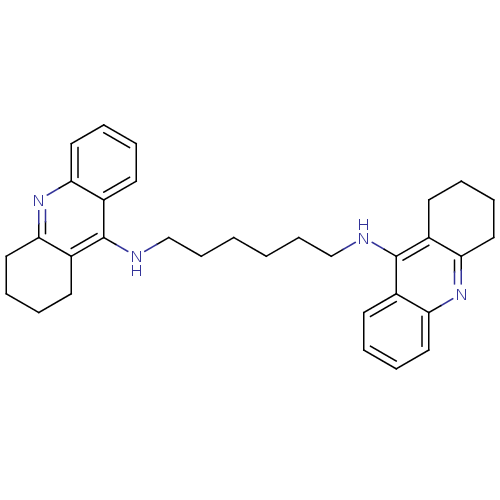 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate measured after 8 mins in presence of BuChE inhibitor ethopropazine by Ellma... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50149212 (CHEMBL117521 | N-(1,2,3,4,8a,10a-Hexahydro-acridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Acetylcholinesterase in rat brain | J Med Chem 47: 3463-82 (2004) Article DOI: 10.1021/jm040031v BindingDB Entry DOI: 10.7270/Q2NC61ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398922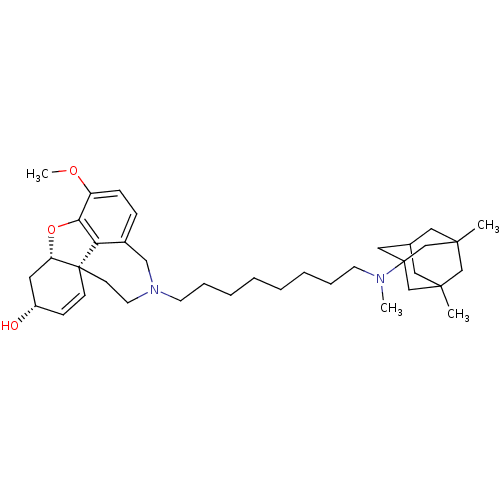 (CHEMBL2178790) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9051 (6-fluoro-N-{7-[(6-fluoro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9053 (6-chloro-N-{6-[(6-chloro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964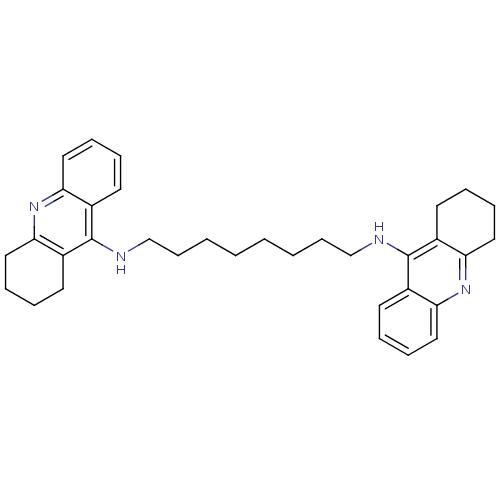 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was determined in vitro and ex vivo for anti-AChE activity in rat brain | Bioorg Med Chem Lett 2: 871-876 (1992) Article DOI: 10.1016/S0960-894X(00)80547-8 BindingDB Entry DOI: 10.7270/Q21R6QDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9052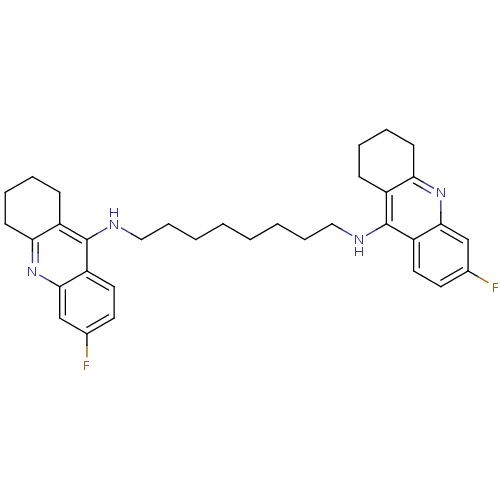 (6-fluoro-N-{8-[(6-fluoro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10469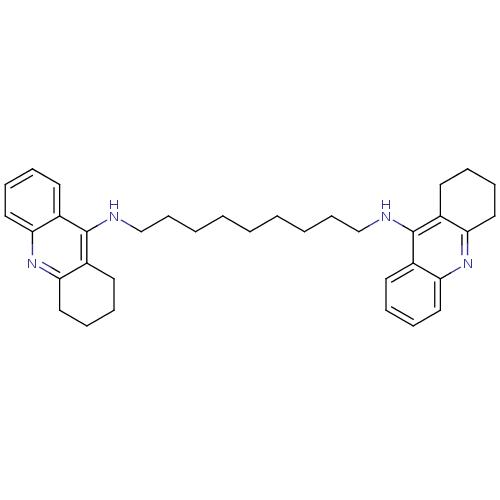 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mayo Foundation for Medical Education and Research | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Biol Chem 271: 23646-9 (1996) Article DOI: 10.1074/jbc.271.39.23646 BindingDB Entry DOI: 10.7270/Q2XW4H0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10469 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m... | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50273221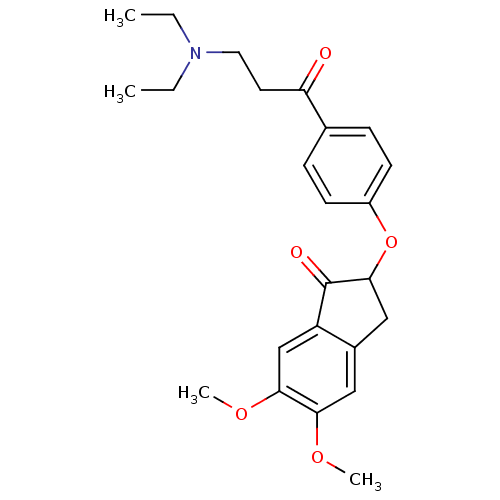 (2-(4-(3-(Diethylamino)propanoyl)phenoxy)-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AchE in rat cortex by Ellman's method | Bioorg Med Chem 16: 7646-53 (2008) Article DOI: 10.1016/j.bmc.2008.07.014 BindingDB Entry DOI: 10.7270/Q2RR1Z2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50568800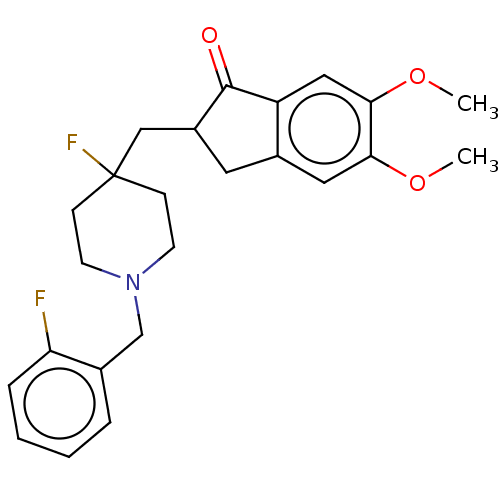 (CHEMBL4859647) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex homogenate acetylcholinesterase using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01863 BindingDB Entry DOI: 10.7270/Q2JW8JNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9050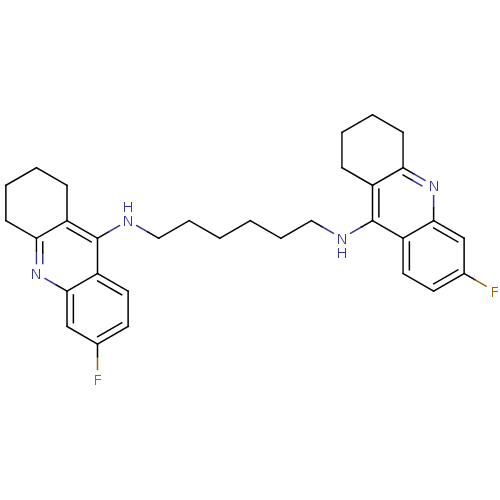 (6-fluoro-N-{6-[(6-fluoro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582079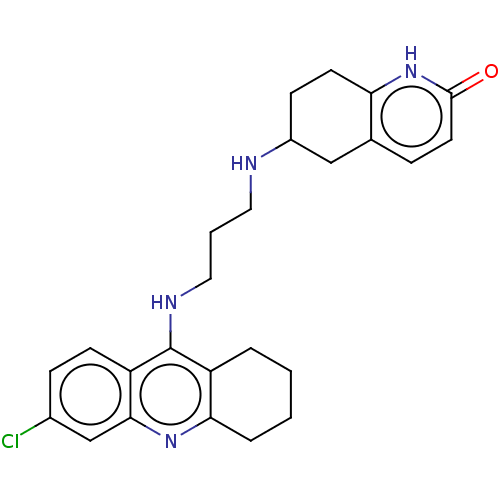 (CHEMBL5091959) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50568792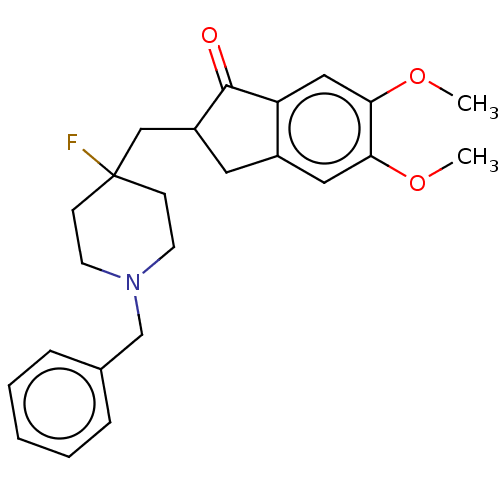 (CHEMBL4846450) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex homogenate acetylcholinesterase using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01863 BindingDB Entry DOI: 10.7270/Q2JW8JNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of rat brain AChE by Ellman's method | Bioorg Med Chem Lett 20: 1532-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.097 BindingDB Entry DOI: 10.7270/Q29K4BCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398924 (CHEMBL2178788) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398928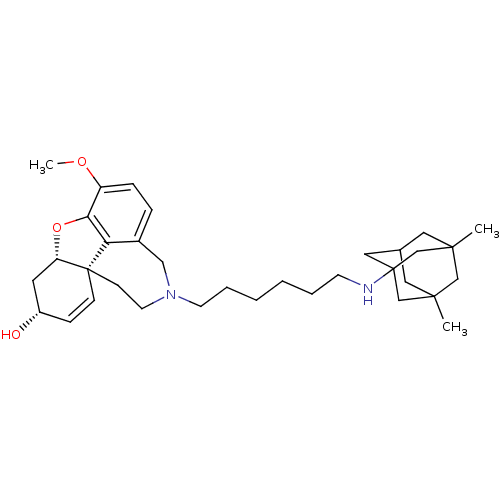 (CHEMBL2178784) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9057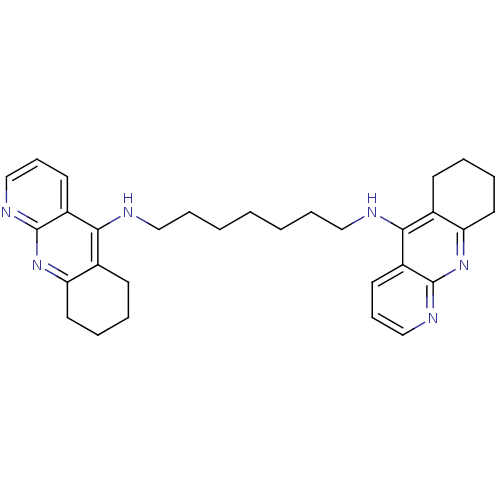 (Homodimeric Tacrine Analog 3k | N,N-Bis-(1,2,3,4-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398923 (CHEMBL2178789) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9047 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of rat AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040619 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(3-nitro-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibition against acetylcholinesterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9426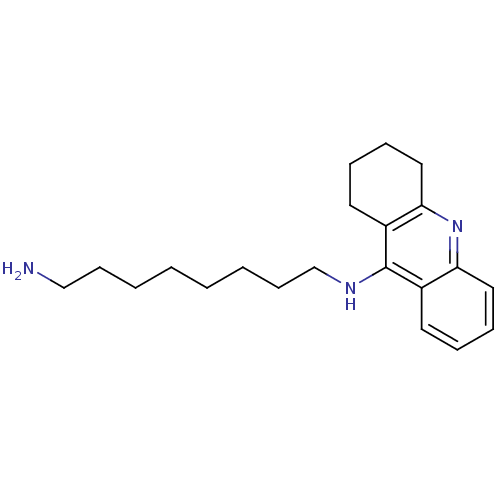 (CHEMBL258928 | Heterodimeric Tacrine-Based Inhibit...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of rat AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 42: 4225-31 (1999) Article DOI: 10.1021/jm990224w BindingDB Entry DOI: 10.7270/Q2W957CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50231951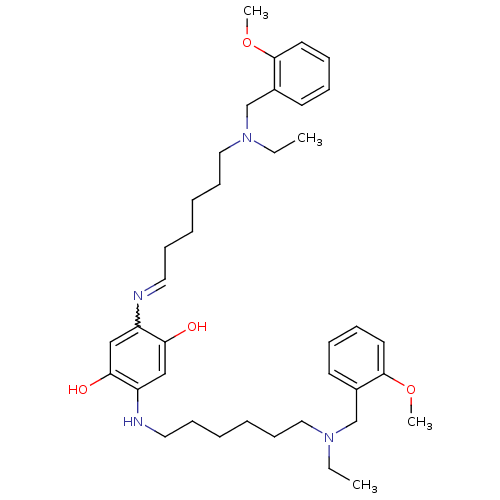 (2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of rat AChE | Bioorg Med Chem 26: 6115-6127 (2018) Article DOI: 10.1016/j.bmc.2018.11.015 BindingDB Entry DOI: 10.7270/Q2WD4400 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9061 (Homodimeric Tacrine Analog 4c | N,N-Bis-(2,3,4,5-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description In vitro inhibitory effect on rat Acetylcholinesterase | J Med Chem 38: 2969-73 (1995) BindingDB Entry DOI: 10.7270/Q22B8X2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398927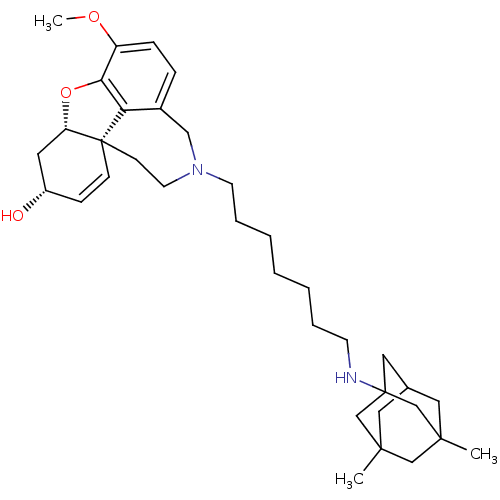 (CHEMBL2178785) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50273222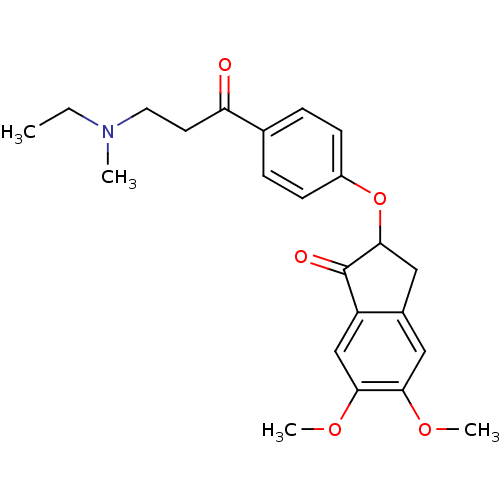 (2-(4-(3-(Ethyl(methyl)amino)propanoyl)phenoxy)-5,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AchE in rat cortex by Ellman's method | Bioorg Med Chem 16: 7646-53 (2008) Article DOI: 10.1016/j.bmc.2008.07.014 BindingDB Entry DOI: 10.7270/Q2RR1Z2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9058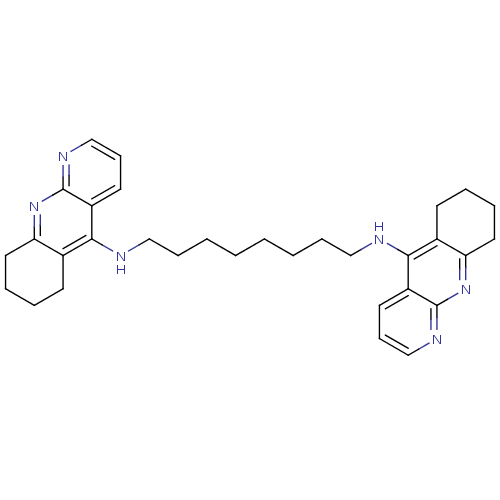 (Homodimeric Tacrine Analog 3m | N,N-Bis-(1,2,3,4-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50040622 (1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(9,10-diox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholine esterase (AChE) | J Med Chem 37: 689-95 (1994) BindingDB Entry DOI: 10.7270/Q2N29W0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50568808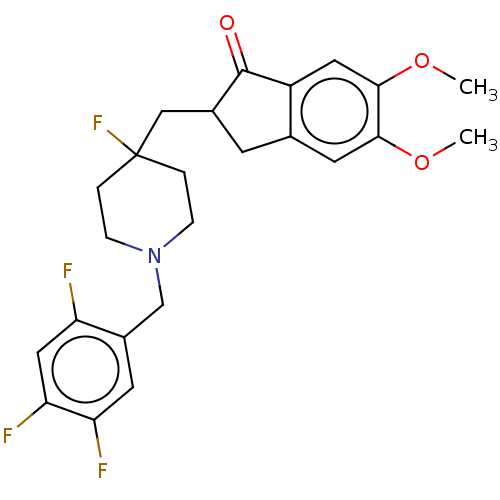 (CHEMBL4857100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex homogenate acetylcholinesterase using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01863 BindingDB Entry DOI: 10.7270/Q2JW8JNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex homogenate acetylcholinesterase using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01863 BindingDB Entry DOI: 10.7270/Q2JW8JNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50284042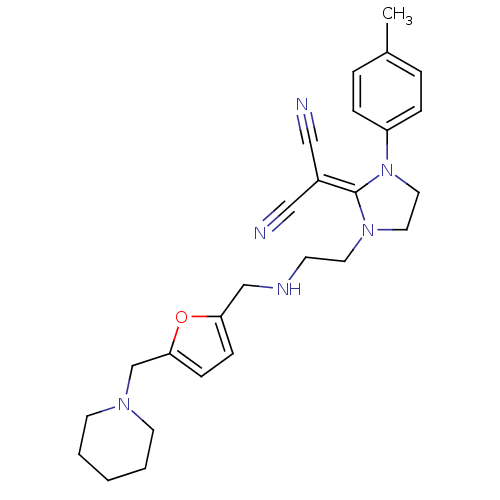 (2-(1-{2-[(5-Piperidin-1-ylmethyl-furan-2-ylmethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against acetylcholinesterase(AChE) from rat brain was determined | Bioorg Med Chem Lett 4: 615-618 (1994) Article DOI: 10.1016/S0960-894X(01)80165-7 BindingDB Entry DOI: 10.7270/Q2FN1659 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50398925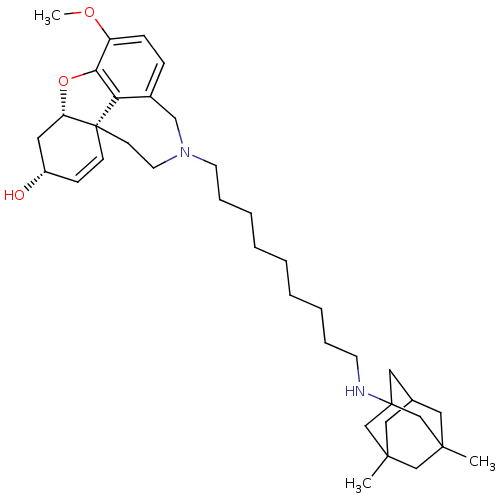 (CHEMBL2178787) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman method | J Med Chem 55: 9708-21 (2012) Article DOI: 10.1021/jm3009458 BindingDB Entry DOI: 10.7270/Q2VQ33TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9059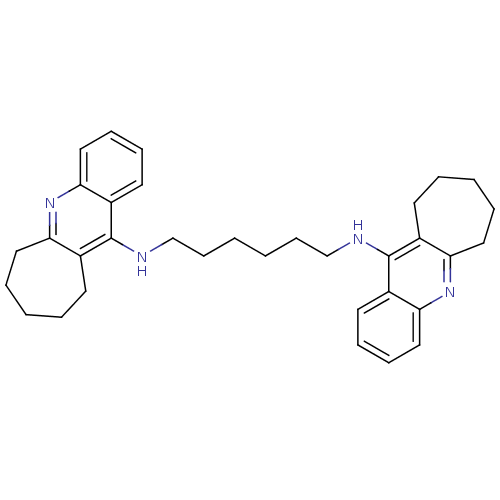 (Homodimeric Tacrine Analog 4a | N,N-Bis-(2,3,4,5-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1713 total ) | Next | Last >> |