

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10440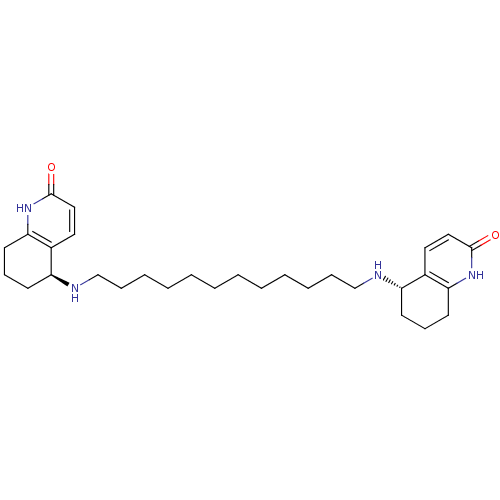 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 19.6 | -10.4 | 52 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of rat AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Wien Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit acetylcholinesterase (AChE) in rat brain; 0.024-0.040 uM | Bioorg Med Chem Lett 11: 2627-30 (2001) BindingDB Entry DOI: 10.7270/Q2VH5PCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 47.1 | -9.89 | 114 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50429847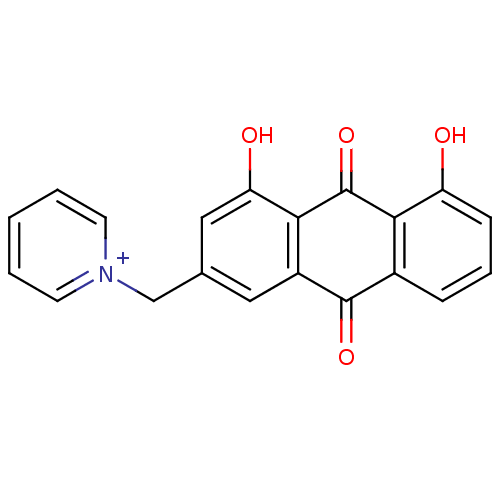 (CHEMBL2338670) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hainan University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex using acetylthiocholine iodide as substrate by double Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 1064-73 (2013) Article DOI: 10.1016/j.bmc.2013.01.015 BindingDB Entry DOI: 10.7270/Q2GB25C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50104605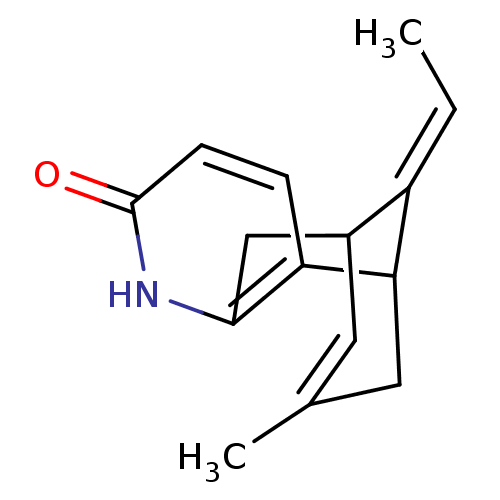 (13-Eth-(E)-ylidene-11-methyl-6-aza-tricyclo[7.3.1....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Wien Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit acetylcholinesterase (AChE) in rat brain | Bioorg Med Chem Lett 11: 2627-30 (2001) BindingDB Entry DOI: 10.7270/Q2VH5PCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026058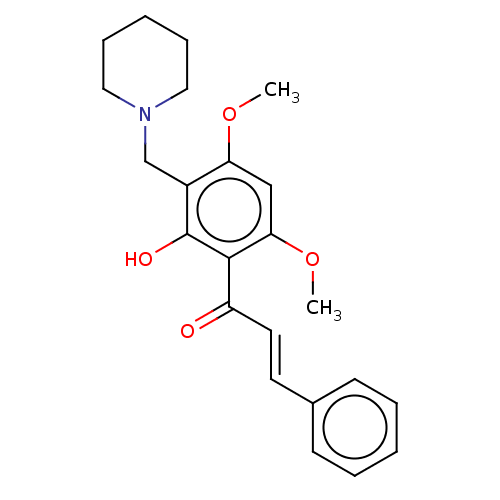 (CHEMBL3335259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE by Michaelis-Menten equation | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026058 (CHEMBL3335259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE using acetylthiocholin iodide as substrate by Michaelis-Menten equation | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50133817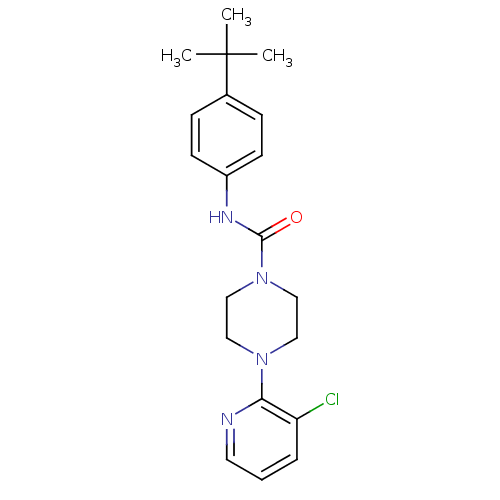 (4-(3-Chloro-pyridin-2-yl)-piperazine-1-carboxylic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pudue Pharma Discovery Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 377-86 (2003) Article DOI: 10.1124/jpet.102.045674 BindingDB Entry DOI: 10.7270/Q2TX3CX5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50104603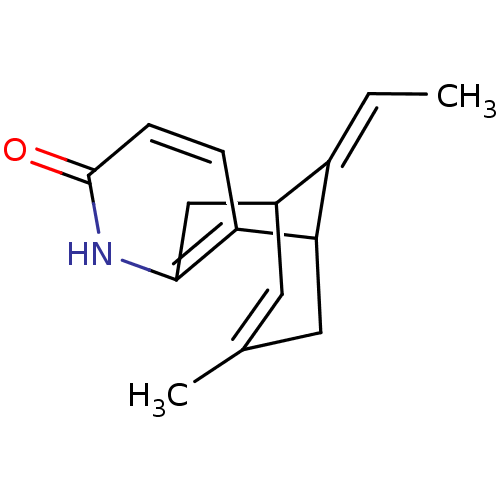 (13-Eth-(Z)-ylidene-11-methyl-6-aza-tricyclo[7.3.1....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Wien Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit acetylcholinesterase (AChE) in rat brain | Bioorg Med Chem Lett 11: 2627-30 (2001) BindingDB Entry DOI: 10.7270/Q2VH5PCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50028409 (CHEMBL285508 | Trimethyl-[3-(2-oxo-2lambda*5*-[1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant of the compound against rat brain acetylcholinesterase at pH 7.0 | J Med Chem 26: 145-52 (1983) BindingDB Entry DOI: 10.7270/Q23T9G75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50104604 (11-Methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Wien Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit acetylcholinesterase (AChE) in rat brain | Bioorg Med Chem Lett 11: 2627-30 (2001) BindingDB Entry DOI: 10.7270/Q2VH5PCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||