 Found 276 hits of kd data for polymerid = 1043,1045,1064,236,3619,3621,50006539,50006985
Found 276 hits of kd data for polymerid = 1043,1045,1064,236,3619,3621,50006539,50006985 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50149201
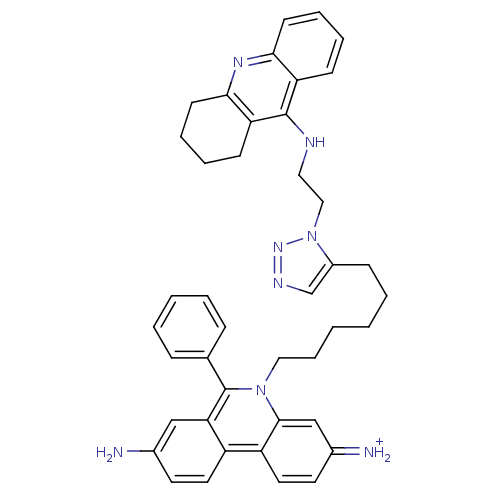
(3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-46-48-50(32)25-23-45-41-35-15-7-9-17-38(35)47-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,47)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles
Curated by ChEMBL
| Assay Description
Binding affinity to acetylcholine esterase (unknown origin) |
Bioorg Med Chem 22: 4474-89 (2014)
Article DOI: 10.1016/j.bmc.2014.04.019
BindingDB Entry DOI: 10.7270/Q29K4CW0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50546265
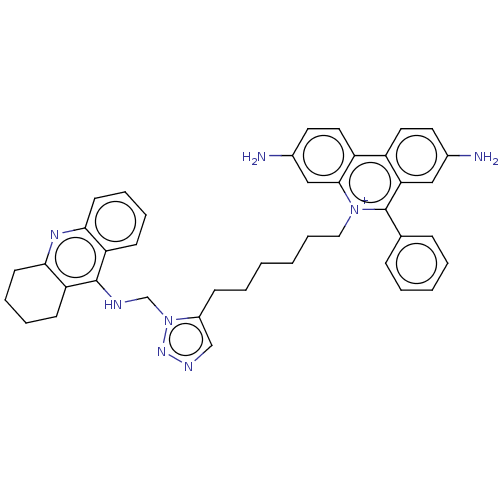
(CHEMBL4753558)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)[n+](CCCCCCc1cnnn1CNc1c3CCCCc3nc3ccccc13)c1cc(N)ccc21 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Torpedo californica acetylcholinesterase by fluorescence method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50044549
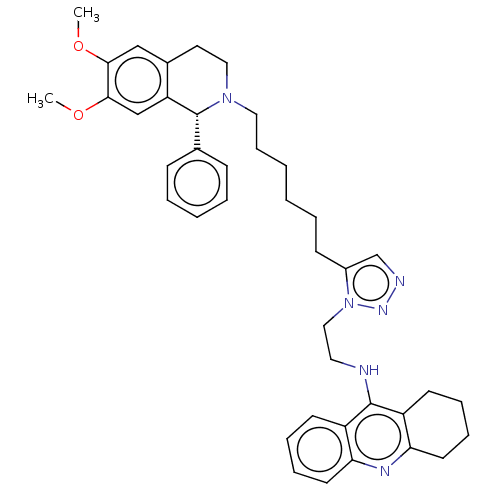
(CHEMBL3314093)Show SMILES COc1cc2CCN(CCCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C40H48N6O2/c1-47-37-26-30-21-24-45(40(29-14-6-5-7-15-29)34(30)27-38(37)48-2)23-13-4-3-8-16-31-28-42-44-46(31)25-22-41-39-32-17-9-11-19-35(32)43-36-20-12-10-18-33(36)39/h5-7,9,11,14-15,17,19,26-28,40H,3-4,8,10,12-13,16,18,20-25H2,1-2H3,(H,41,43)/t40-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles
Curated by ChEMBL
| Assay Description
Binding affinity to acetylcholine esterase (unknown origin) |
Bioorg Med Chem 22: 4474-89 (2014)
Article DOI: 10.1016/j.bmc.2014.04.019
BindingDB Entry DOI: 10.7270/Q29K4CW0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50044549

(CHEMBL3314093)Show SMILES COc1cc2CCN(CCCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C40H48N6O2/c1-47-37-26-30-21-24-45(40(29-14-6-5-7-15-29)34(30)27-38(37)48-2)23-13-4-3-8-16-31-28-42-44-46(31)25-22-41-39-32-17-9-11-19-35(32)43-36-20-12-10-18-33(36)39/h5-7,9,11,14-15,17,19,26-28,40H,3-4,8,10,12-13,16,18,20-25H2,1-2H3,(H,41,43)/t40-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50462576
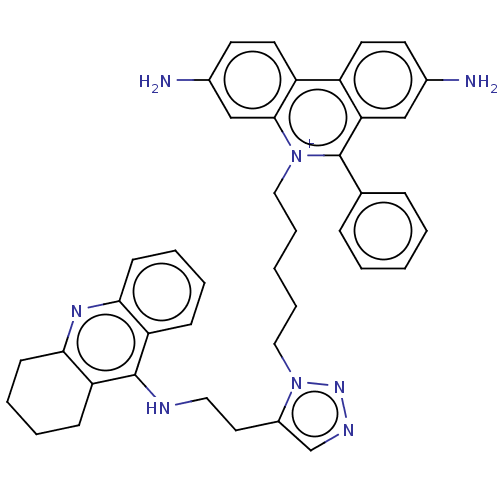
(CHEMBL4249808)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)[n+](CCCCCn1nncc1CCNc1c3CCCCc3nc3ccccc13)c1cc(N)ccc21 Show InChI InChI=1S/C41H42N8/c42-29-17-19-32-33-20-18-30(43)26-39(33)48(41(36(32)25-29)28-11-3-1-4-12-28)23-9-2-10-24-49-31(27-45-47-49)21-22-44-40-34-13-5-7-15-37(34)46-38-16-8-6-14-35(38)40/h1,3-5,7,11-13,15,17-20,25-27,43H,2,6,8-10,14,16,21-24,42H2,(H,44,46)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50462577
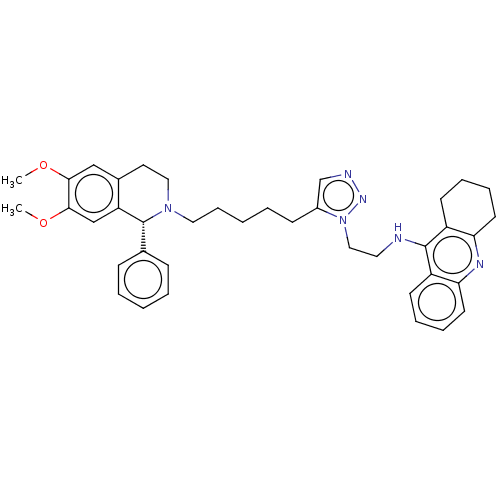
(CHEMBL4243833)Show SMILES COc1cc2CCN(CCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C39H46N6O2/c1-46-36-25-29-20-23-44(39(28-13-5-3-6-14-28)33(29)26-37(36)47-2)22-12-4-7-15-30-27-41-43-45(30)24-21-40-38-31-16-8-10-18-34(31)42-35-19-11-9-17-32(35)38/h3,5-6,8,10,13-14,16,18,25-27,39H,4,7,9,11-12,15,17,19-24H2,1-2H3,(H,40,42)/t39-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50462578
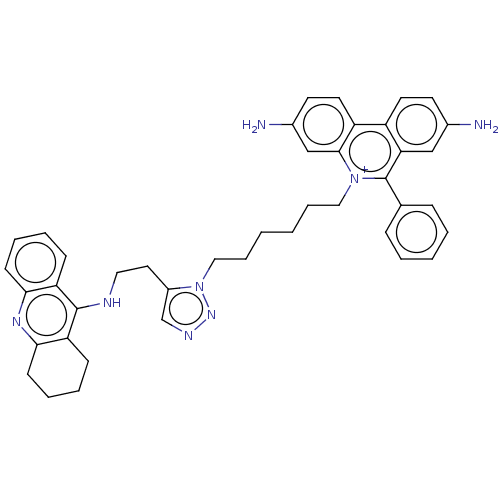
(CHEMBL4239697)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)[n+](CCCCCCn1nncc1CCNc1c3CCCCc3nc3ccccc13)c1cc(N)ccc21 Show InChI InChI=1S/C42H44N8/c43-30-18-20-33-34-21-19-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-10-1-2-11-25-50-32(28-46-48-50)22-23-45-41-35-14-6-8-16-38(35)47-39-17-9-7-15-36(39)41/h3-6,8,12-14,16,18-21,26-28,44H,1-2,7,9-11,15,17,22-25,43H2,(H,45,47)/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50462580
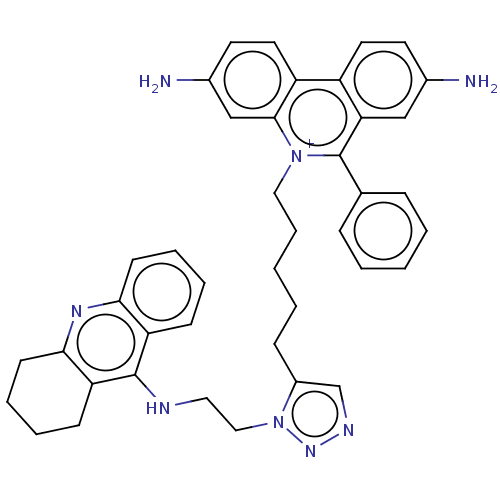
(CHEMBL4239268)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)[n+](CCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(N)ccc21 Show InChI InChI=1S/C41H42N8/c42-29-18-20-32-33-21-19-30(43)26-39(33)48(41(36(32)25-29)28-11-3-1-4-12-28)23-10-2-5-13-31-27-45-47-49(31)24-22-44-40-34-14-6-8-16-37(34)46-38-17-9-7-15-35(38)40/h1,3-4,6,8,11-12,14,16,18-21,25-27,43H,2,5,7,9-10,13,15,17,22-24,42H2,(H,44,46)/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50044549

(CHEMBL3314093)Show SMILES COc1cc2CCN(CCCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C40H48N6O2/c1-47-37-26-30-21-24-45(40(29-14-6-5-7-15-29)34(30)27-38(37)48-2)23-13-4-3-8-16-31-28-42-44-46(31)25-22-41-39-32-17-9-11-19-35(32)43-36-20-12-10-18-33(36)39/h5-7,9,11,14-15,17,19,26-28,40H,3-4,8,10,12-13,16,18,20-25H2,1-2H3,(H,41,43)/t40-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50462577

(CHEMBL4243833)Show SMILES COc1cc2CCN(CCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C39H46N6O2/c1-46-36-25-29-20-23-44(39(28-13-5-3-6-14-28)33(29)26-37(36)47-2)22-12-4-7-15-30-27-41-43-45(30)24-21-40-38-31-16-8-10-18-34(31)42-35-19-11-9-17-32(35)38/h3,5-6,8,10,13-14,16,18,25-27,39H,4,7,9,11-12,15,17,19-24H2,1-2H3,(H,40,42)/t39-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50462581

(CHEMBL4246013)Show SMILES COc1cc2CCN(CCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C39H46N6O2/c1-46-36-25-29-20-23-44(39(28-13-5-3-6-14-28)33(29)26-37(36)47-2)22-12-4-7-15-30-27-41-43-45(30)24-21-40-38-31-16-8-10-18-34(31)42-35-19-11-9-17-32(35)38/h3,5-6,8,10,13-14,16,18,25-27,39H,4,7,9,11-12,15,17,19-24H2,1-2H3,(H,40,42)/t39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50462578

(CHEMBL4239697)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)[n+](CCCCCCn1nncc1CCNc1c3CCCCc3nc3ccccc13)c1cc(N)ccc21 Show InChI InChI=1S/C42H44N8/c43-30-18-20-33-34-21-19-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-10-1-2-11-25-50-32(28-46-48-50)22-23-45-41-35-14-6-8-16-38(35)47-39-17-9-7-15-36(39)41/h3-6,8,12-14,16,18-21,26-28,44H,1-2,7,9-11,15,17,22-25,43H2,(H,45,47)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50462580

(CHEMBL4239268)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)[n+](CCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(N)ccc21 Show InChI InChI=1S/C41H42N8/c42-29-18-20-32-33-21-19-30(43)26-39(33)48(41(36(32)25-29)28-11-3-1-4-12-28)23-10-2-5-13-31-27-45-47-49(31)24-22-44-40-34-14-6-8-16-37(34)46-38-17-9-7-15-35(38)40/h1,3-4,6,8,11-12,14,16,18-21,25-27,43H,2,5,7,9-10,13,15,17,22-24,42H2,(H,44,46)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50462581

(CHEMBL4246013)Show SMILES COc1cc2CCN(CCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C39H46N6O2/c1-46-36-25-29-20-23-44(39(28-13-5-3-6-14-28)33(29)26-37(36)47-2)22-12-4-7-15-30-27-41-43-45(30)24-21-40-38-31-16-8-10-18-34(31)42-35-19-11-9-17-32(35)38/h3,5-6,8,10,13-14,16,18,25-27,39H,4,7,9,11-12,15,17,19-24H2,1-2H3,(H,40,42)/t39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50044548
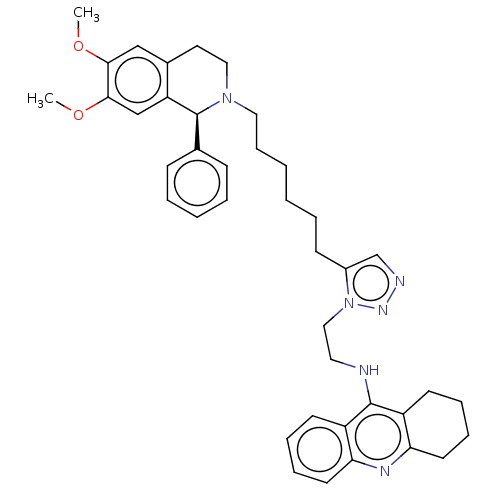
(CHEMBL3314092)Show SMILES COc1cc2CCN(CCCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C40H48N6O2/c1-47-37-26-30-21-24-45(40(29-14-6-5-7-15-29)34(30)27-38(37)48-2)23-13-4-3-8-16-31-28-42-44-46(31)25-22-41-39-32-17-9-11-19-35(32)43-36-20-12-10-18-33(36)39/h5-7,9,11,14-15,17,19,26-28,40H,3-4,8,10,12-13,16,18,20-25H2,1-2H3,(H,41,43)/t40-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50462576

(CHEMBL4249808)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)[n+](CCCCCn1nncc1CCNc1c3CCCCc3nc3ccccc13)c1cc(N)ccc21 Show InChI InChI=1S/C41H42N8/c42-29-17-19-32-33-20-18-30(43)26-39(33)48(41(36(32)25-29)28-11-3-1-4-12-28)23-9-2-10-24-49-31(27-45-47-49)21-22-44-40-34-13-5-7-15-37(34)46-38-16-8-6-14-35(38)40/h1,3-5,7,11-13,15,17-20,25-27,43H,2,6,8-10,14,16,21-24,42H2,(H,44,46)/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50149201

(3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-46-48-50(32)25-23-45-41-35-15-7-9-17-38(35)47-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,47)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50044548

(CHEMBL3314092)Show SMILES COc1cc2CCN(CCCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C40H48N6O2/c1-47-37-26-30-21-24-45(40(29-14-6-5-7-15-29)34(30)27-38(37)48-2)23-13-4-3-8-16-31-28-42-44-46(31)25-22-41-39-32-17-9-11-19-35(32)43-36-20-12-10-18-33(36)39/h5-7,9,11,14-15,17,19,26-28,40H,3-4,8,10,12-13,16,18,20-25H2,1-2H3,(H,41,43)/t40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of mouse AChE active site after 12 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50149201

(3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-46-48-50(32)25-23-45-41-35-15-7-9-17-38(35)47-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,47)/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50044548

(CHEMBL3314092)Show SMILES COc1cc2CCN(CCCCCCc3cnnn3CCNc3c4CCCCc4nc4ccccc34)[C@@H](c3ccccc3)c2cc1OC |r| Show InChI InChI=1S/C40H48N6O2/c1-47-37-26-30-21-24-45(40(29-14-6-5-7-15-29)34(30)27-38(37)48-2)23-13-4-3-8-16-31-28-42-44-46(31)25-22-41-39-32-17-9-11-19-35(32)43-36-20-12-10-18-33(36)39/h5-7,9,11,14-15,17,19,26-28,40H,3-4,8,10,12-13,16,18,20-25H2,1-2H3,(H,41,43)/t40-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles
Curated by ChEMBL
| Assay Description
Binding affinity to acetylcholine esterase (unknown origin) |
Bioorg Med Chem 22: 4474-89 (2014)
Article DOI: 10.1016/j.bmc.2014.04.019
BindingDB Entry DOI: 10.7270/Q29K4CW0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50149201

(3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-46-48-50(32)25-23-45-41-35-15-7-9-17-38(35)47-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,47)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.000410 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant towards Acetylcholinesterase in mouse |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556952

(CHEMBL4754487)Show SMILES COc1cc(CNC(=O)CCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:34,TLB:23:22:19:34.31.32,THB:16:17:19:34.31.32| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed-type inhibition of recombinant human AChE using varying concentration of acetylthiocholine iodide as substrate at 0.17 to 1.36 nM by Lineweaver... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50568793
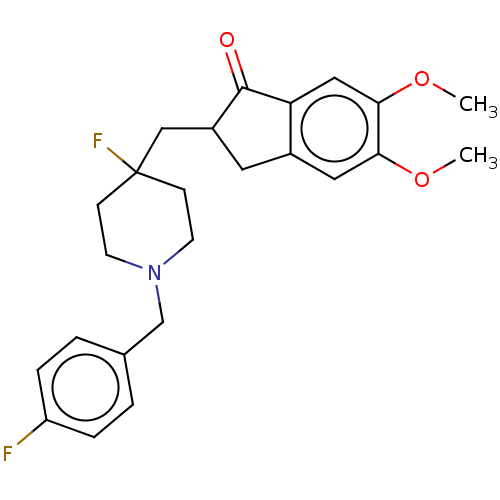
(CHEMBL4857791)Show SMILES COc1cc2CC(CC3(F)CCN(Cc4ccc(F)cc4)CC3)C(=O)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CM5 sensor chip immobilized recombinant human AChE assessed as dissociation constant at 298.15 K by surface plasmon resonance ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01863
BindingDB Entry DOI: 10.7270/Q2JW8JNQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50568804
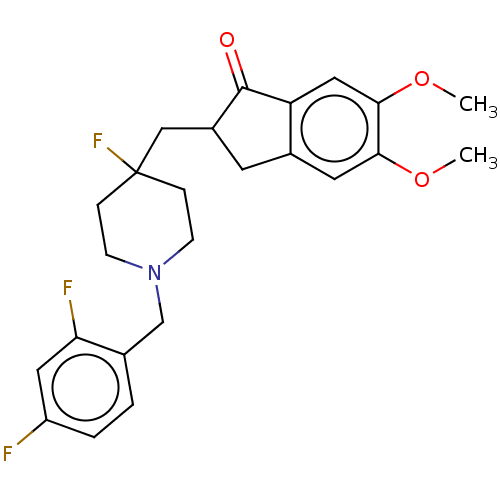
(CHEMBL4877719)Show SMILES COc1cc2CC(CC3(F)CCN(Cc4ccc(F)cc4F)CC3)C(=O)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CM5 sensor chip immobilized recombinant human AChE assessed as dissociation constant at 298.15 K by surface plasmon resonance ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01863
BindingDB Entry DOI: 10.7270/Q2JW8JNQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50568800
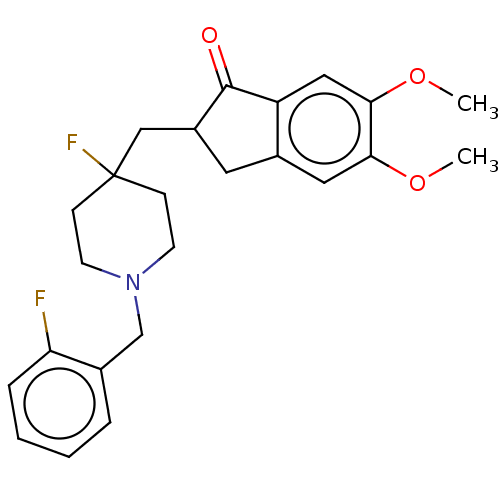
(CHEMBL4859647)Show SMILES COc1cc2CC(CC3(F)CCN(Cc4ccccc4F)CC3)C(=O)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CM5 sensor chip immobilized recombinant human AChE assessed as dissociation constant at 298.15 K by surface plasmon resonance ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01863
BindingDB Entry DOI: 10.7270/Q2JW8JNQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50556952

(CHEMBL4754487)Show SMILES COc1cc(CNC(=O)CCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:34,TLB:23:22:19:34.31.32,THB:16:17:19:34.31.32| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of propidium iodide from PAS-region of electric eel AChE assessed as dissociation constant by spectrofluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50568792
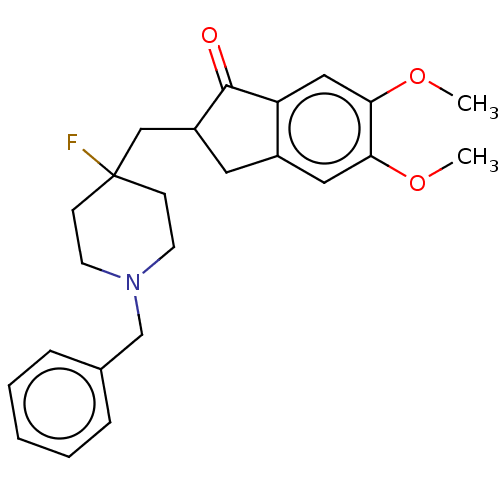
(CHEMBL4846450)Show SMILES COc1cc2CC(CC3(F)CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CM5 sensor chip immobilized recombinant human AChE assessed as dissociation constant at 298.15 K by surface plasmon resonance ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01863
BindingDB Entry DOI: 10.7270/Q2JW8JNQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50568795
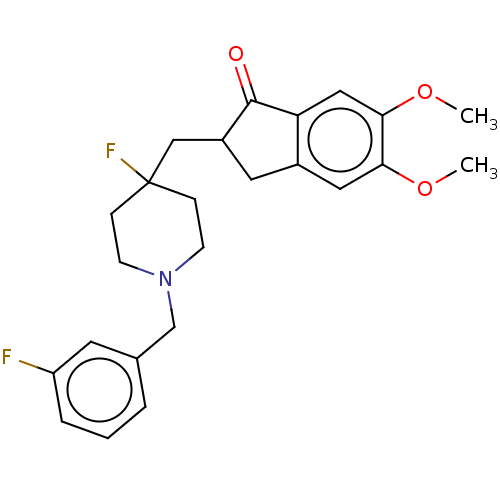
(CHEMBL4853698)Show SMILES COc1cc2CC(CC3(F)CCN(Cc4cccc(F)c4)CC3)C(=O)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CM5 sensor chip immobilized recombinant human AChE assessed as dissociation constant at 298.15 K by surface plasmon resonance ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01863
BindingDB Entry DOI: 10.7270/Q2JW8JNQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50568808
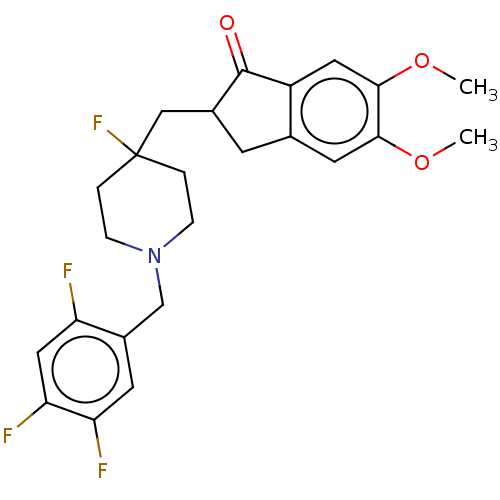
(CHEMBL4857100)Show SMILES COc1cc2CC(CC3(F)CCN(Cc4cc(F)c(F)cc4F)CC3)C(=O)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CM5 sensor chip immobilized recombinant human AChE assessed as dissociation constant at 298.15 K by surface plasmon resonance ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01863
BindingDB Entry DOI: 10.7270/Q2JW8JNQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CM5 sensor chip immobilized recombinant human AChE assessed as dissociation constant at 298.15 K by surface plasmon resonance ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01863
BindingDB Entry DOI: 10.7270/Q2JW8JNQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50546263
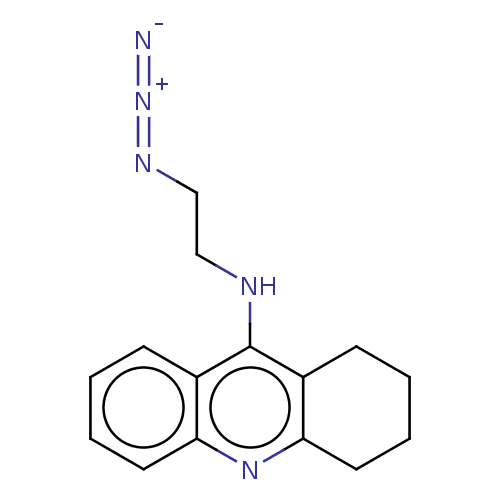
(CHEMBL4749021) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to mouse ACHE by fluorescence method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50199522

((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@@]1(N)CC(C)=C2 |r,c:18,THB:1:2:14.15.17:5.11.4| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CM5 sensor chip immobilized recombinant human AChE assessed as dissociation constant at 298.15 K by surface plasmon resonance ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01863
BindingDB Entry DOI: 10.7270/Q2JW8JNQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant towards Acetylcholinesterase in mouse |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50292572
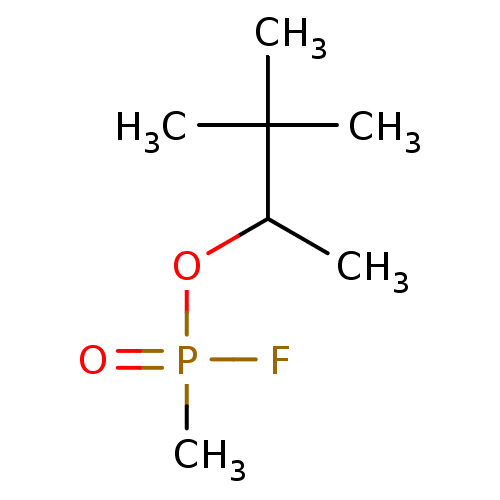
(3,3-dimethylbutan-2-yl methylphosphonofluoridate |...)Show InChI InChI=1S/C7H16FO2P/c1-6(7(2,3)4)10-11(5,8)9/h6H,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptor from rat brain membranes using [3H]cyclohexyladenosine as radioligand. |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50292572

(3,3-dimethylbutan-2-yl methylphosphonofluoridate |...)Show InChI InChI=1S/C7H16FO2P/c1-6(7(2,3)4)10-11(5,8)9/h6H,1-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50563478
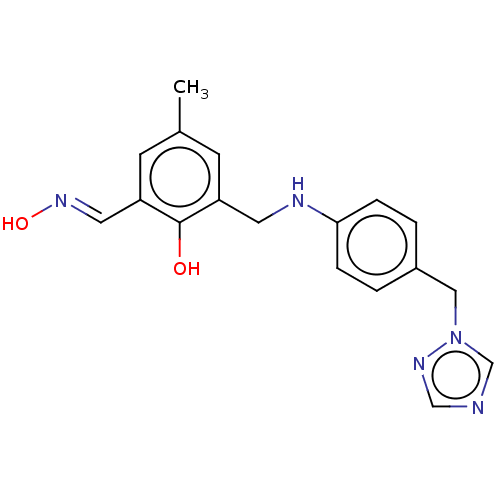
(CHEMBL4752879) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reactivation of paraoxon-inhibited human AChE assessed as dissociation constant incubated for 30 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113286
BindingDB Entry DOI: 10.7270/Q2ZP49VN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50563478

(CHEMBL4752879) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reactivation of parathion-inhibited human AChE assessed as dissociation constant incubated for 30 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113286
BindingDB Entry DOI: 10.7270/Q2ZP49VN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50563478

(CHEMBL4752879) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reactivation of dichlorvos-inhibited human AChE assessed as dissociation constant incubated for 30 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113286
BindingDB Entry DOI: 10.7270/Q2ZP49VN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50462582
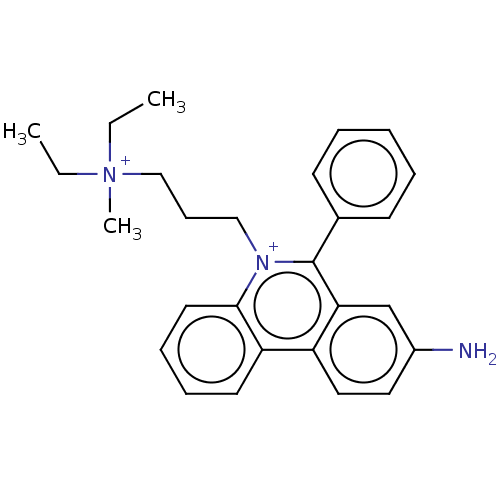
(CHEMBL4250783)Show SMILES CC[N+](C)(CC)CCC[n+]1c(-c2ccccc2)c2cc(N)ccc2c2ccccc12 Show InChI InChI=1S/C27H33N3/c1-4-30(3,5-2)19-11-18-29-26-15-10-9-14-24(26)23-17-16-22(28)20-25(23)27(29)21-12-7-6-8-13-21/h6-10,12-17,20H,4-5,11,18-19,28H2,1-3H3/q+2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity to electric eel AChE after 24 hrs by LC/MS-SIM analysis |
J Med Chem 61: 9395-9409 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00266
BindingDB Entry DOI: 10.7270/Q2765J0C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005563
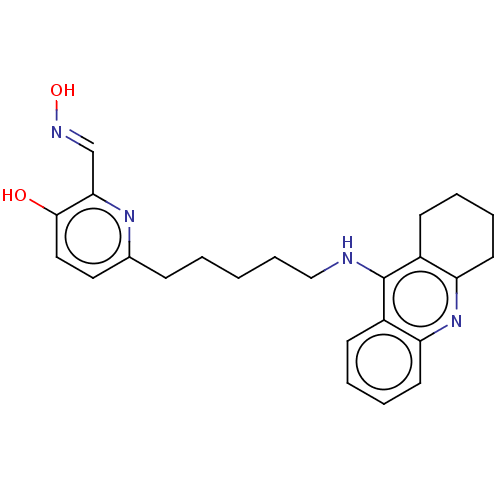
(CHEMBL3234587 | CHEMBL434456)Show SMILES O\N=C\c1nc(CCCCCNc2c3CCCCc3nc3ccccc23)ccc1O Show InChI InChI=1S/C24H28N4O2/c29-23-14-13-17(27-22(23)16-26-30)8-2-1-7-15-25-24-18-9-3-5-11-20(18)28-21-12-6-4-10-19(21)24/h3,5,9,11,13-14,16,29-30H,1-2,4,6-8,10,12,15H2,(H,25,28)/b26-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of paraoxon-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM31904
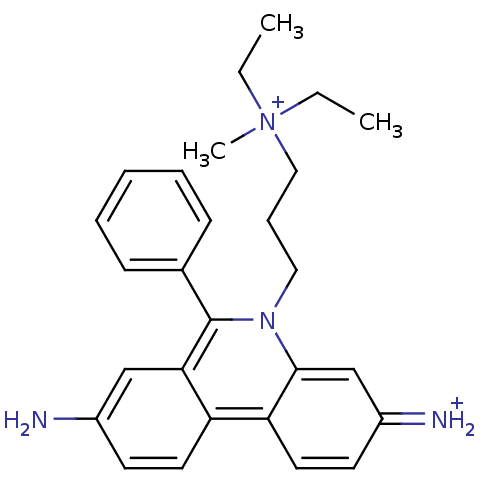
(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant towards Acetylcholinesterase in mouse |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005579
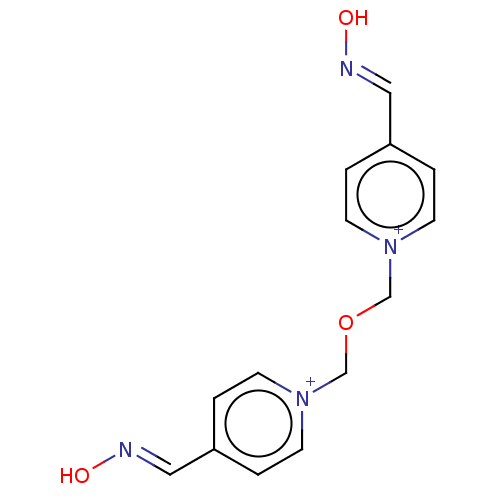
(CHEMBL139148 | Obidoxime)Show InChI InChI=1S/C14H14N4O3/c19-15-9-13-1-5-17(6-2-13)11-21-12-18-7-3-14(4-8-18)10-16-20/h1-10H,11-12H2/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reactivation of dichlorvos-inhibited human AChE assessed as dissociation constant incubated for 30 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113286
BindingDB Entry DOI: 10.7270/Q2ZP49VN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
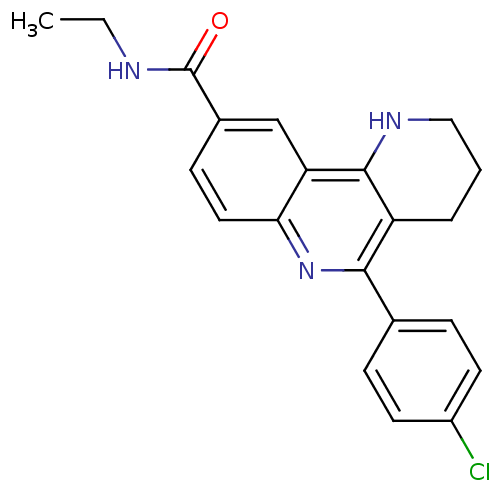
(Electrophorus electricus (Electric eel)) | BDBM50448131
(CHEMBL3122167)Show SMILES CCNC(=O)c1ccc2nc(-c3ccc(Cl)cc3)c3CCCNc3c2c1 Show InChI InChI=1S/C21H20ClN3O/c1-2-23-21(26)14-7-10-18-17(12-14)20-16(4-3-11-24-20)19(25-18)13-5-8-15(22)9-6-13/h5-10,12,24H,2-4,11H2,1H3,(H,23,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Displacement of propidium from electric eel AChE peripheral anionic site assessed as decrease in fluorescence intensity by spectrofluorometric analys... |
Eur J Med Chem 73: 141-52 (2014)
Article DOI: 10.1016/j.ejmech.2013.12.008
BindingDB Entry DOI: 10.7270/Q2CF9RKN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
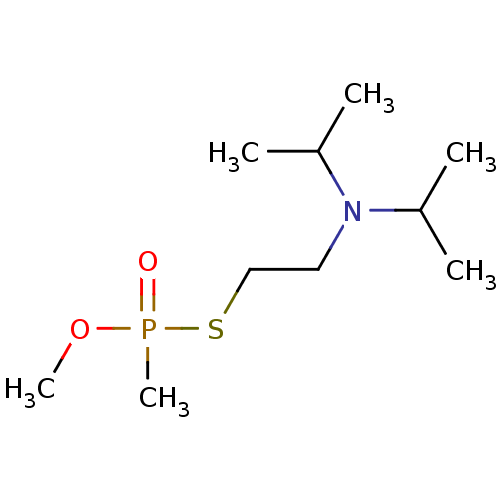
(Electrophorus electricus (Electric eel)) | BDBM50241991
(CHEMBL4085438)Show InChI InChI=1S/C10H24NO2PS/c1-9(2)11(10(3)4)7-8-15-14(6,12)13-5/h9-10H,7-8H2,1-6H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Binding affinity to Electric eel AChE |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
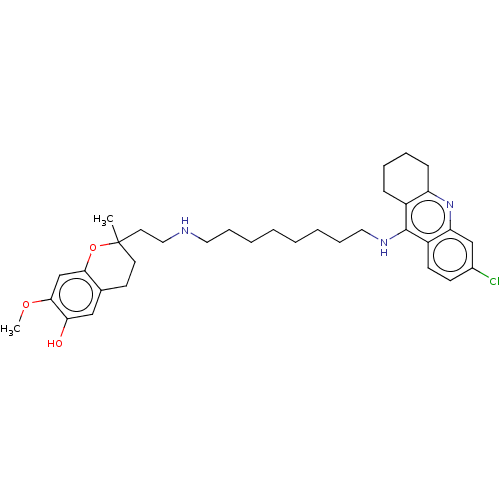
(Electrophorus electricus (Electric eel)) | BDBM50560224
(CHEMBL4751100)Show SMILES COc1cc2OC(C)(CCNCCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)CCc2cc1O | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of propidium iodide from PAS region of electric eel AChE by fluorescence assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00528
BindingDB Entry DOI: 10.7270/Q23N2733 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005579

(CHEMBL139148 | Obidoxime)Show InChI InChI=1S/C14H14N4O3/c19-15-9-13-1-5-17(6-2-13)11-21-12-18-7-3-14(4-8-18)10-16-20/h1-10H,11-12H2/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reactivation of sarin-inhibited human AChE assessed as dissociation constant incubated for 30 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113286
BindingDB Entry DOI: 10.7270/Q2ZP49VN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50448130
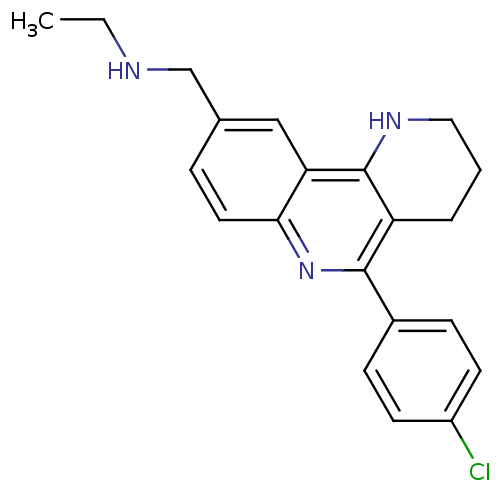
(CHEMBL3122168)Show InChI InChI=1S/C21H22ClN3/c1-2-23-13-14-5-10-19-18(12-14)21-17(4-3-11-24-21)20(25-19)15-6-8-16(22)9-7-15/h5-10,12,23-24H,2-4,11,13H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Displacement of propidium from electric eel AChE peripheral anionic site assessed as decrease in fluorescence intensity by spectrofluorometric analys... |
Eur J Med Chem 73: 141-52 (2014)
Article DOI: 10.1016/j.ejmech.2013.12.008
BindingDB Entry DOI: 10.7270/Q2CF9RKN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50027343
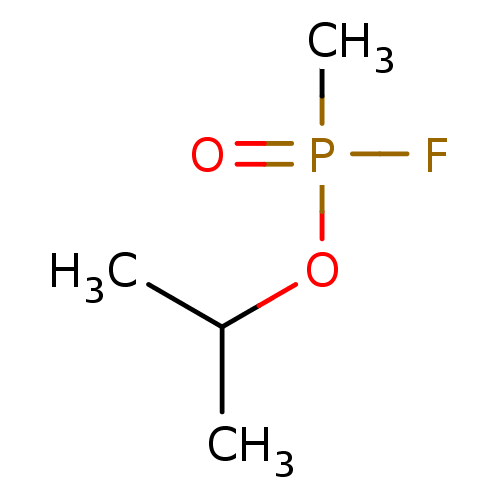
(CHEBI:75701 | ISOPROPYL METHYLPHOSPHONOFLUORIDATE)Show InChI InChI=1S/C4H10FO2P/c1-4(2)7-8(3,5)6/h4H,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Binding affinity to Electric eel AChE |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50187216
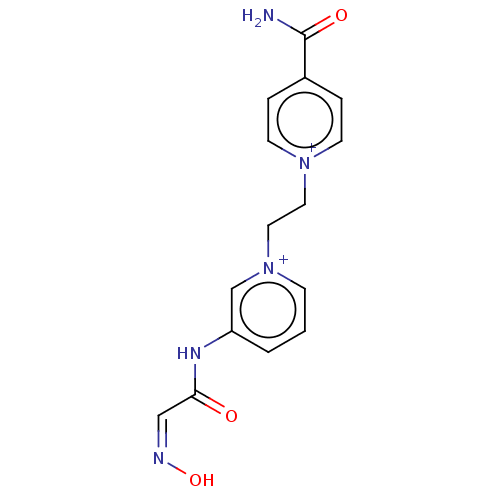
(CHEMBL3827575)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CC[n+]2cccc(NC(=O)\C=N/O)c2)cc1 Show InChI InChI=1S/C15H15N5O3.2BrH/c16-15(22)12-3-6-19(7-4-12)8-9-20-5-1-2-13(11-20)18-14(21)10-17-23;;/h1-7,10-11H,8-9H2,(H2-2,16,18,21,22,23);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a |
Defence Research& Development Establishment
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free erythrocyte ghost human AChE using acetylcholine iodide as substrate measured for 1 hr by spectro... |
Bioorg Med Chem 24: 4171-4176 (2016)
Article DOI: 10.1016/j.bmc.2016.07.005
BindingDB Entry DOI: 10.7270/Q25D8TSK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data