

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117587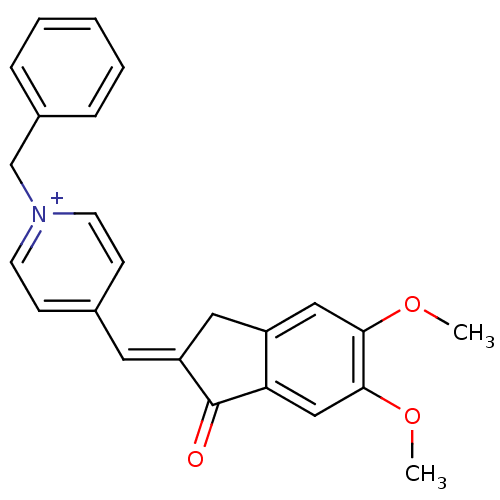 (1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylidenemet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117594 (1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50199518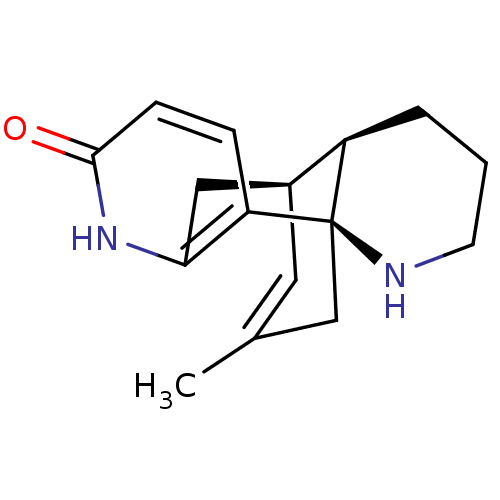 ((-)-huperzine B | CHEMBL245079 | huperzine B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117577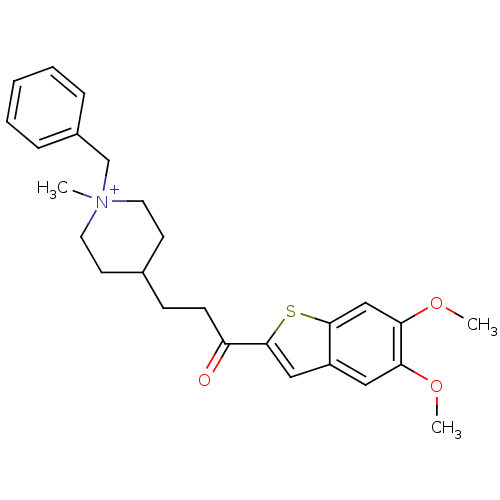 (1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10958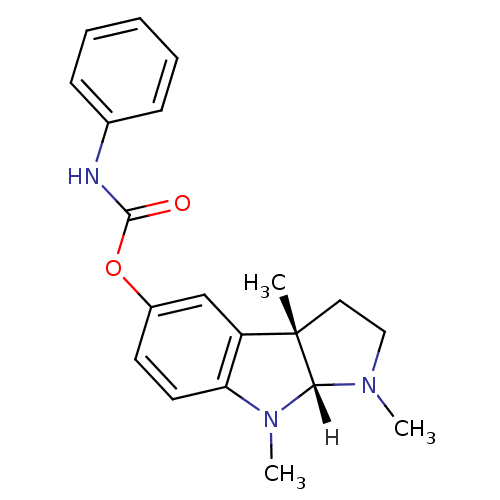 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Aristea Translational Medicine Corporation US Patent | Assay Description To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To... | US Patent US10864192 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM476178 ((−)-N8-norphenylcarbamoyleseroline | US10864...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Aristea Translational Medicine Corporation US Patent | Assay Description To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To... | US Patent US10864192 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM476174 ((−)-N1-norphenylcarbamoyleseroline | US10864...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Aristea Translational Medicine Corporation US Patent | Assay Description To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To... | US Patent US10864192 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117584 (1-Benzyl-4-[3-(2,3-dihydro-benzofuran-5-yl)-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117595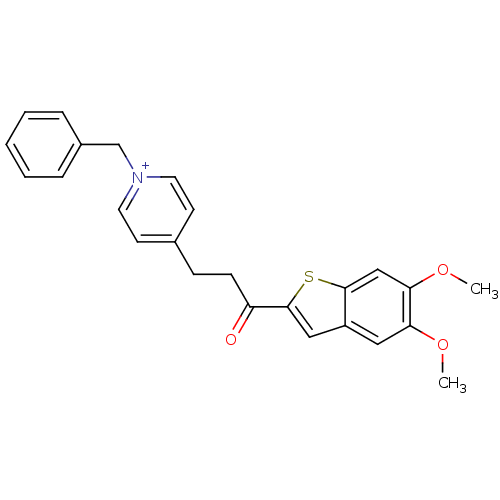 (1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM476179 ((−)-N1, N8-bisnorphenylcarbamoyleseroline | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Aristea Translational Medicine Corporation US Patent | Assay Description To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To... | US Patent US10864192 (2020) BindingDB Entry DOI: 10.7270/Q2TT4V23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117592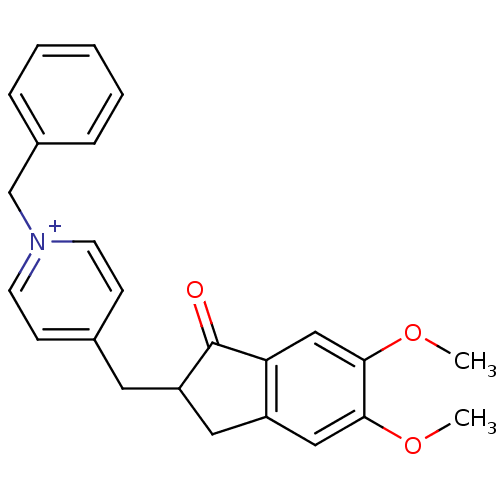 (1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117583 (1-Benzyl-4-(5,6-dimethoxy-1-oxo-indan-2-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117599 (1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117620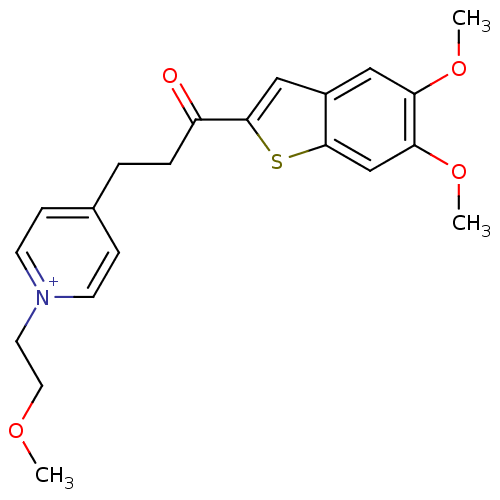 (4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117580 (1-Benzyl-1-methyl-4-[3-oxo-3-(2,3,4,5-tetrahydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 331 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117574 (1-Benzyl-1-methyl-4-[3-(5-methyl-1-phenyl-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117576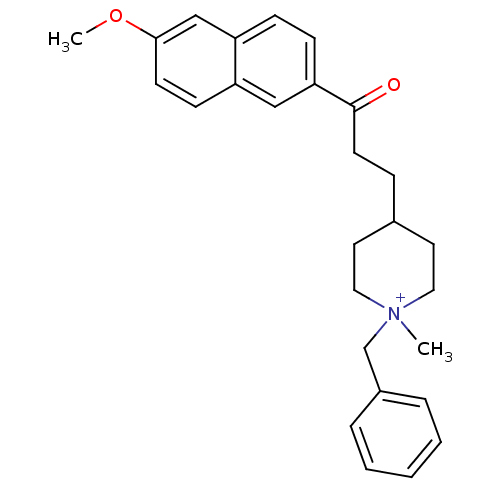 (1-Benzyl-4-[3-(6-methoxy-naphthalen-2-yl)-3-oxo-pr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117582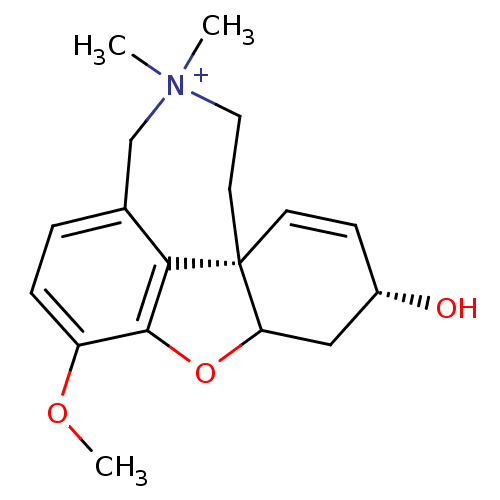 (9-methoxy-4,4-dimethyl-(1S,14R)-11-oxa-4-azoniatet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117615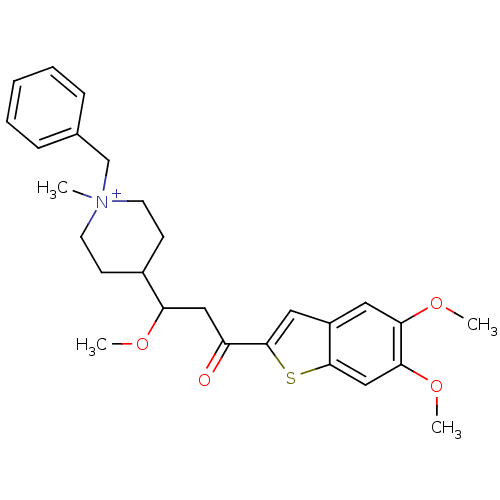 (1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 650 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117590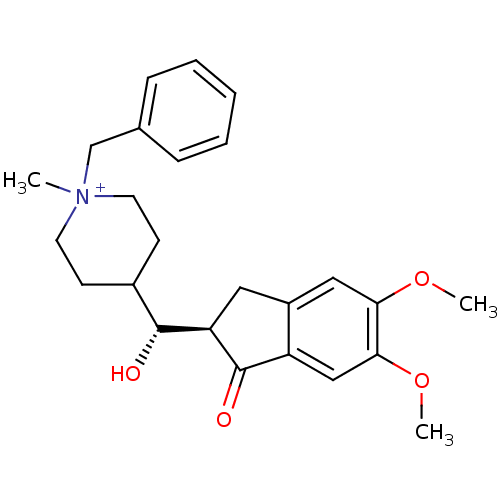 (1-Benzyl-4-[(5,6-dimethoxy-1-oxo-indan-2-yl)-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 890 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50037157 (3-(1-Benzyl-piperidin-4-yl)-1-(2,3,4,5-tetrahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117589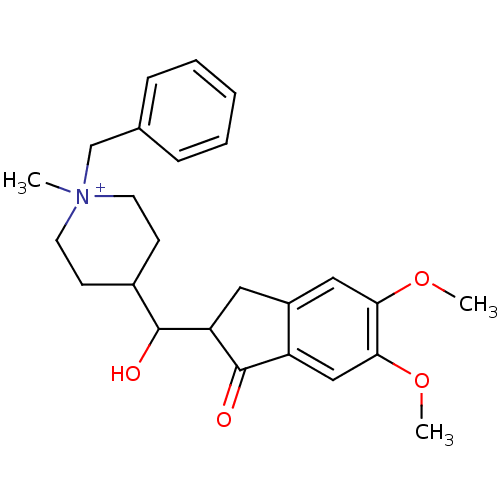 (1-Benzyl-4-[(5,6-dimethoxy-1-oxo-indan-2-yl)-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117585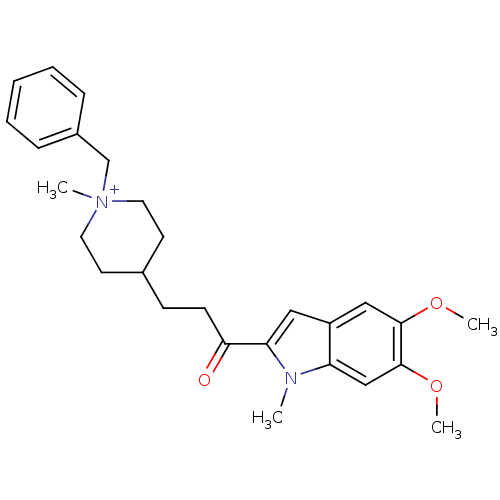 (1-Benzyl-4-[3-(5,6-dimethoxy-1-methyl-1H-indol-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117612 (4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117617 (4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117591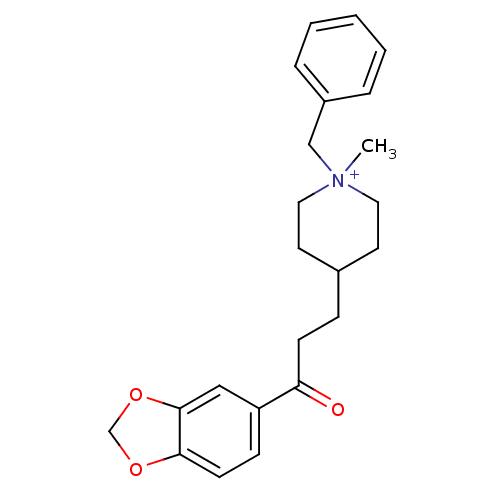 (4-(3-Benzo[1,3]dioxol-5-yl-3-oxo-propyl)-1-benzyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117623 (1-Cyclobutylmethyl-4-[3-(5,6-dimethoxy-benzo[b]thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117593 (1-Benzyl-4-[(5,6-dimethoxy-1-oxo-indan-2-yl)-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117604 (4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50366796 (CHEMBL609440) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117614 (4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117575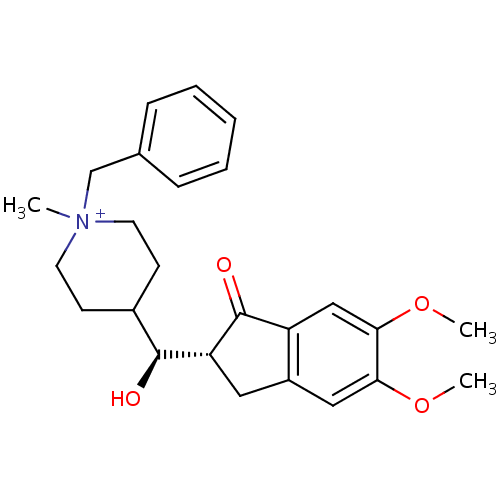 (1-Benzyl-4-[(5,6-dimethoxy-1-oxo-indan-2-yl)-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117603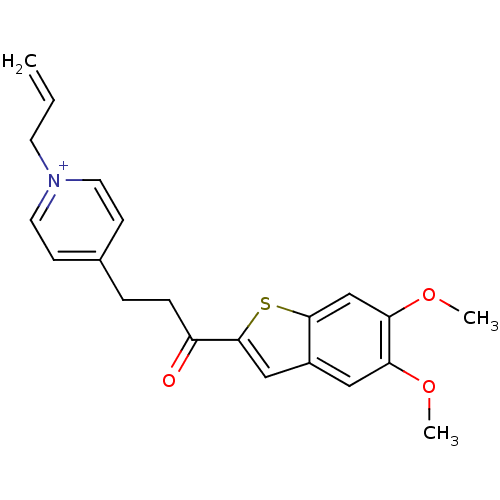 (1-Allyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117596 (1-Cyclopropylmethyl-4-[3-(5,6-dimethoxy-benzo[b]th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117578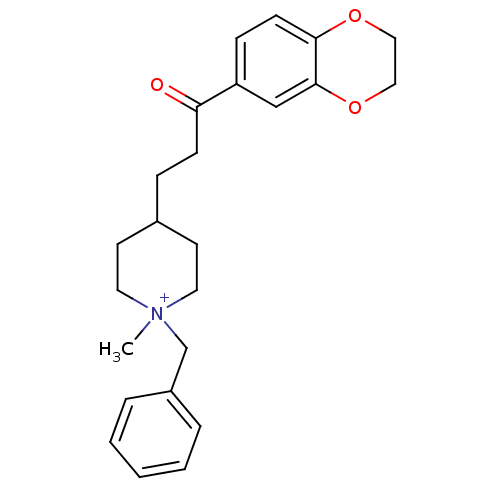 (1-Benzyl-4-[3-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117605 (4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117600 (1-Benzyl-4-[5-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117616 (1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.67E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117608 (1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 9.43E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117606 (1-Benzyl-4-[5-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117573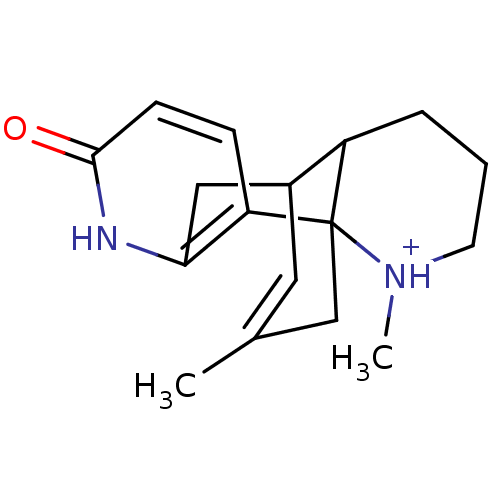 (14,16-dimethyl-14-azonia-6-azatetracyclo[7.5.3.01,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50516235 (CHEMBL4563408) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Activity at human recombinant ACHE using acetylthiocholine as substrate measured after 30 mins by spectrophotometric assay | J Med Chem 62: 6330-6345 (2019) Article DOI: 10.1021/acs.jmedchem.9b00727 BindingDB Entry DOI: 10.7270/Q24F1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117607 (1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117610 (1-Benzyl-4-[(5,6-dimethoxy-benzo[b]thiophen-2-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117601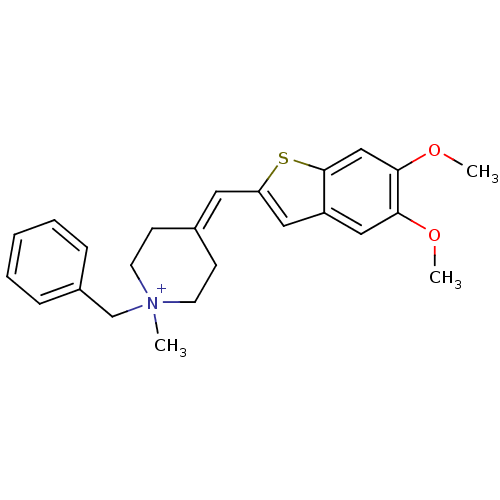 (1-Benzyl-4-(5,6-dimethoxy-benzo[b]thiophen-2-ylmet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117621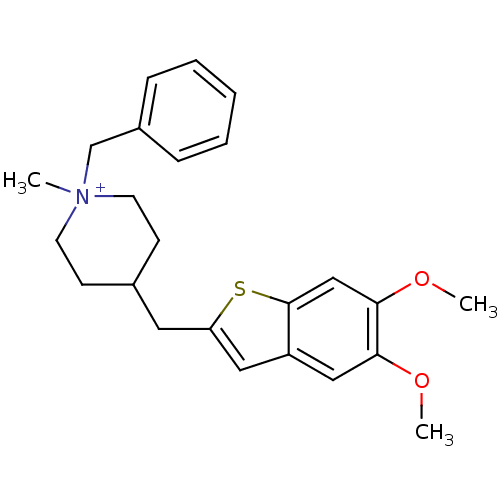 (1-Benzyl-4-(5,6-dimethoxy-benzo[b]thiophen-2-ylmet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description Concentration required for the inhibition of acetylcholinesterase | Bioorg Med Chem Lett 12: 2569-72 (2002) BindingDB Entry DOI: 10.7270/Q28916C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50117586 (1,1-Dibenzyl-4-[(5,6-dimethoxy-1-oxo-indan-2-yl)-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.35E+4 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. | Bioorg Med Chem Lett 12: 2565-8 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||