 Found 5431 hits of ic50 data for polymerid = 13,1558,3933,50001868
Found 5431 hits of ic50 data for polymerid = 13,1558,3933,50001868 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6469
(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc(Nc2c(cnc3cc4cc(OCCN5CCOCC5)c(OC)cc4cc23)C#N)c(C)cc1Cl Show InChI InChI=1S/C29H29ClN4O4/c1-18-10-23(30)26(35-2)15-24(18)33-29-21(16-31)17-32-25-12-20-14-28(27(36-3)13-19(20)11-22(25)29)38-9-6-34-4-7-37-8-5-34/h10-15,17H,4-9H2,1-3H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
J Med Chem 48: 5909-20 (2005)
Article DOI: 10.1021/jm050512u
BindingDB Entry DOI: 10.7270/Q2833Q6N |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50197690
(CHEMBL3976548)Show SMILES Nc1c(cnn1-c1cc(O)ccc1Cl)C(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C18H13ClN4O2/c19-13-6-5-11(24)8-16(13)23-18(20)12(9-21-23)17(25)15-7-10-3-1-2-4-14(10)22-15/h1-9,22,24H,20H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SRC by time-resolved fluorescence or time-resolved fluorescence assay |
J Med Chem 59: 10586-10600 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01156
BindingDB Entry DOI: 10.7270/Q2ZS2ZGV |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114442
BindingDB Entry DOI: 10.7270/Q2Z89HCM |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6479
(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc2cc3c(Nc4cc(OC)c(OC)c(OC)c4)c(cnc3cc2cc1OCCN1CCOCC1)C#N Show InChI InChI=1S/C30H32N4O6/c1-35-25-13-19-11-23-24(12-20(19)14-26(25)40-10-7-34-5-8-39-9-6-34)32-18-21(17-31)29(23)33-22-15-27(36-2)30(38-4)28(16-22)37-3/h11-16,18H,5-10H2,1-4H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2989-92 (2002)
Article DOI: 10.1016/s0960-894x(02)00577-2
BindingDB Entry DOI: 10.7270/Q24B2ZHC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244622
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES CN1CCN(CC1)c1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C28H34N7OP/c1-20-7-6-8-21(2)24(20)13-14-35-19-29-25-26(30-22-9-11-23(12-10-22)37(4,5)36)31-28(32-27(25)35)34-17-15-33(3)16-18-34/h6-14,19H,15-18H2,1-5H3,(H,30,31,32)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244621
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)nc(nc12)N1CCOCC1 Show InChI InChI=1S/C27H31N6O2P/c1-19-6-5-7-20(2)23(19)12-13-33-18-28-24-25(29-21-8-10-22(11-9-21)36(3,4)34)30-27(31-26(24)33)32-14-16-35-17-15-32/h5-13,18H,14-17H2,1-4H3,(H,29,30,31)/b13-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM14691
(4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylphe...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)c(C=O)c1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C31H34N3O8P/c1-21(36)32-28(18-23-12-15-29(26(17-23)20-35)42-43(39,40)41)30(37)33-27-9-5-6-16-34(31(27)38)19-22-10-13-25(14-11-22)24-7-3-2-4-8-24/h2-4,7-8,10-15,17,20,27-28H,5-6,9,16,18-19H2,1H3,(H,32,36)(H,33,37)(H2,39,40,41)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Binding affinity for Src SH2 domain in scintillation proximity binding assay (SPA) |
J Med Chem 45: 2379-87 (2002)
BindingDB Entry DOI: 10.7270/Q21J992G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src [145-252]
(Homo sapiens (Human)) | BDBM14691

(4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylphe...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)c(C=O)c1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C31H34N3O8P/c1-21(36)32-28(18-23-12-15-29(26(17-23)20-35)42-43(39,40)41)30(37)33-27-9-5-6-16-34(31(27)38)19-22-10-13-25(14-11-22)24-7-3-2-4-8-24/h2-4,7-8,10-15,17,20,27-28H,5-6,9,16,18-19H2,1H3,(H,32,36)(H,33,37)(H2,39,40,41)/t27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
SPA uses 125I as energy donor and scintillant-coated beads as energy acceptor. The labeled ligand is captured by the biotinylated Src-SH2 protein imm... |
J Med Chem 46: 5184-95 (2003)
Article DOI: 10.1021/jm020970s
BindingDB Entry DOI: 10.7270/Q2XW4H14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM14691

(4-[(2S)-2-acetamido-2-{[(3S)-2-oxo-1-[(4-phenylphe...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)c(C=O)c1)C(=O)N[C@H]1CCCCN(Cc2ccc(cc2)-c2ccccc2)C1=O |r| Show InChI InChI=1S/C31H34N3O8P/c1-21(36)32-28(18-23-12-15-29(26(17-23)20-35)42-43(39,40)41)30(37)33-27-9-5-6-16-34(31(27)38)19-22-10-13-25(14-11-22)24-7-3-2-4-8-24/h2-4,7-8,10-15,17,20,27-28H,5-6,9,16,18-19H2,1H3,(H,32,36)(H,33,37)(H2,39,40,41)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory concentration against SH2 domain of human p60 Src tyrosine kinase |
J Med Chem 45: 2915-22 (2002)
BindingDB Entry DOI: 10.7270/Q2ZW1MNM |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6472
(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc(Nc2c(cnc3cc4cc(OCCN5CCN(C)CC5)c(OC)cc4cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C29H29Cl2N5O3/c1-35-4-6-36(7-5-35)8-9-39-28-13-19-11-24-21(10-18(19)12-27(28)38-3)29(20(16-32)17-33-24)34-25-15-26(37-2)23(31)14-22(25)30/h10-15,17H,4-9H2,1-3H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
J Med Chem 48: 5909-20 (2005)
Article DOI: 10.1021/jm050512u
BindingDB Entry DOI: 10.7270/Q2833Q6N |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132351
(({4-[6-(2,6-Dichloro-phenyl)-8-(3-dimethylamino-pr...)Show SMILES CN(C)CCCn1c2nc(Nc3ccc(cc3)P(O)(=O)CP(O)(O)=O)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(3.9,-7.22,;3.9,-5.68,;2.57,-4.91,;5.23,-4.91,;5.23,-3.37,;6.56,-2.59,;6.56,-1.05,;5.21,-.28,;3.88,-1.05,;2.55,-.29,;1.22,-1.06,;-.11,-.29,;-.11,1.25,;-1.44,2.01,;-2.78,1.23,;-2.77,-.31,;-1.44,-1.07,;-4.12,2,;-4.89,.67,;-3.35,3.34,;-5.45,2.77,;-5.45,4.31,;-6.99,4.32,;-5.86,5.8,;-4.12,5.09,;2.55,1.26,;3.88,2.03,;5.21,1.26,;6.54,2.03,;7.89,1.25,;9.22,2.02,;9.22,3.57,;7.89,4.33,;10.55,4.36,;11.88,3.57,;11.88,2.02,;10.55,1.25,;10.55,-.29,;7.89,-.28,;9.22,-1.05,)| Show InChI InChI=1S/C25H27Cl2N5O6P2/c1-31(2)11-4-12-32-23-16(13-19(24(32)33)22-20(26)5-3-6-21(22)27)14-28-25(30-23)29-17-7-9-18(10-8-17)39(34,35)15-40(36,37)38/h3,5-10,13-14H,4,11-12,15H2,1-2H3,(H,34,35)(H,28,29,30)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6470
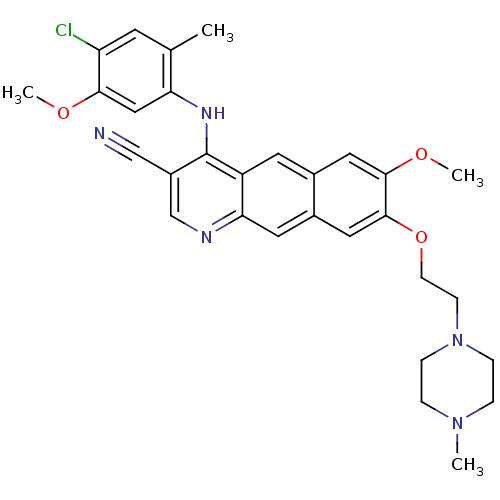
(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc(Nc2c(cnc3cc4cc(OCCN5CCN(C)CC5)c(OC)cc4cc23)C#N)c(C)cc1Cl Show InChI InChI=1S/C30H32ClN5O3/c1-19-11-24(31)27(37-3)16-25(19)34-30-22(17-32)18-33-26-13-21-15-29(28(38-4)14-20(21)12-23(26)30)39-10-9-36-7-5-35(2)6-8-36/h11-16,18H,5-10H2,1-4H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
J Med Chem 48: 5909-20 (2005)
Article DOI: 10.1021/jm050512u
BindingDB Entry DOI: 10.7270/Q2833Q6N |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50414005
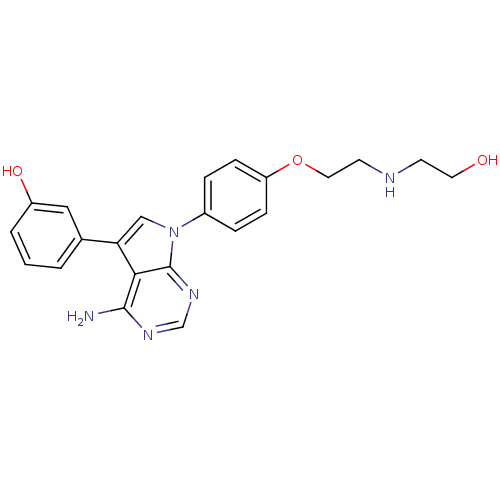
(CHEMBL474998)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)-c1ccc(OCCNCCO)cc1 Show InChI InChI=1S/C22H23N5O3/c23-21-20-19(15-2-1-3-17(29)12-15)13-27(22(20)26-14-25-21)16-4-6-18(7-5-16)30-11-9-24-8-10-28/h1-7,12-14,24,28-29H,8-11H2,(H2,23,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of c-Src |
Eur J Med Chem 44: 990-1000 (2009)
Article DOI: 10.1016/j.ejmech.2008.07.002
BindingDB Entry DOI: 10.7270/Q2ZC8434 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50414006
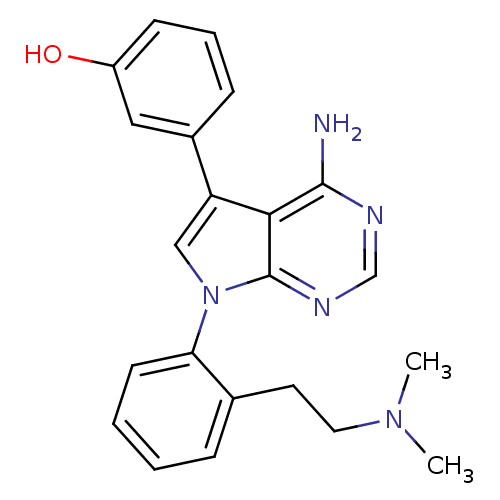
(CHEMBL516062)Show SMILES CN(C)CCc1ccccc1-n1cc(-c2cccc(O)c2)c2c(N)ncnc12 Show InChI InChI=1S/C22H23N5O/c1-26(2)11-10-15-6-3-4-9-19(15)27-13-18(16-7-5-8-17(28)12-16)20-21(23)24-14-25-22(20)27/h3-9,12-14,28H,10-11H2,1-2H3,(H2,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of c-Src |
Eur J Med Chem 44: 990-1000 (2009)
Article DOI: 10.1016/j.ejmech.2008.07.002
BindingDB Entry DOI: 10.7270/Q2ZC8434 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50414005

(CHEMBL474998)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)-c1ccc(OCCNCCO)cc1 Show InChI InChI=1S/C22H23N5O3/c23-21-20-19(15-2-1-3-17(29)12-15)13-27(22(20)26-14-25-21)16-4-6-18(7-5-16)30-11-9-24-8-10-28/h1-7,12-14,24,28-29H,8-11H2,(H2,23,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Src kinase (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-012-0308-3
BindingDB Entry DOI: 10.7270/Q2BZ68ZH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50414006

(CHEMBL516062)Show SMILES CN(C)CCc1ccccc1-n1cc(-c2cccc(O)c2)c2c(N)ncnc12 Show InChI InChI=1S/C22H23N5O/c1-26(2)11-10-15-6-3-4-9-19(15)27-13-18(16-7-5-8-17(28)12-16)20-21(23)24-14-25-22(20)27/h3-9,12-14,28H,10-11H2,1-2H3,(H2,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-Src kinase (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-012-0308-3
BindingDB Entry DOI: 10.7270/Q2BZ68ZH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6474
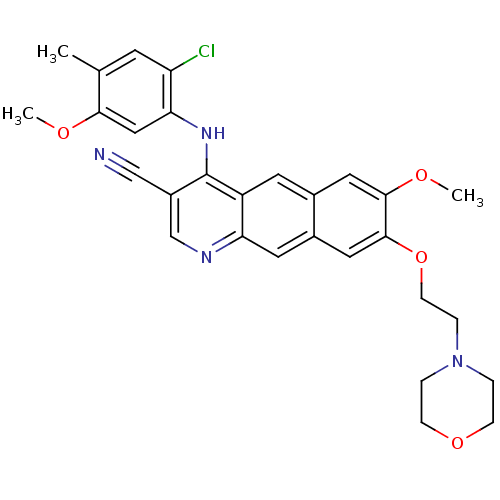
(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc(Nc2c(cnc3cc4cc(OCCN5CCOCC5)c(OC)cc4cc23)C#N)c(Cl)cc1C Show InChI InChI=1S/C29H29ClN4O4/c1-18-10-23(30)25(15-26(18)35-2)33-29-21(16-31)17-32-24-12-20-14-28(27(36-3)13-19(20)11-22(24)29)38-9-6-34-4-7-37-8-5-34/h10-15,17H,4-9H2,1-3H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
J Med Chem 48: 5909-20 (2005)
Article DOI: 10.1021/jm050512u
BindingDB Entry DOI: 10.7270/Q2833Q6N |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.400 | 11 | n/a | n/a | n/a | 7.0 | 23 |
Chemical Genomics Centre of the Max Planck Society
| Assay Description
IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla... |
J Med Chem 52: 3915-26 (2009)
Article DOI: 10.1021/jm9002928
BindingDB Entry DOI: 10.7270/Q2VT1QD3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.400 | 11 | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society
| Assay Description
Tyrosine kinase inhibition assay using wild type cSrc measured by a fluorescence-labeled approach. |
Nat Chem Biol 5: 394-6 (2009)
Article DOI: 10.1038/nchembio.162
BindingDB Entry DOI: 10.7270/Q20V8B46 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50413999
(CGP77675 | CHEMBL475584)Show SMILES COc1cccc(c1)-c1cn(-c2ccc(CCN3CCC(O)CC3)cc2)c2ncnc(N)c12 Show InChI InChI=1S/C26H29N5O2/c1-33-22-4-2-3-19(15-22)23-16-31(26-24(23)25(27)28-17-29-26)20-7-5-18(6-8-20)9-12-30-13-10-21(32)11-14-30/h2-8,15-17,21,32H,9-14H2,1H3,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 22 |
Ankara University
| Assay Description
Kinase activity (Src-family kinases, Hck, Lyn, Fyn, and c-Src) and the effect of the molecules were determined by ProFluor Src-Family Kinase Assay Ki... |
J Enzyme Inhib Med Chem 28: 1080-7 (2013)
Article DOI: 10.3109/14756366.2012.715288
BindingDB Entry DOI: 10.7270/Q2KD1WTX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50197709
(CHEMBL3984621)Show SMILES Nc1c(cnn1-c1cc(O)ccc1Cl)C(=O)c1cc2cc(CN3CCOCC3)ccc2[nH]1 Show InChI InChI=1S/C23H22ClN5O3/c24-18-3-2-16(30)11-21(18)29-23(25)17(12-26-29)22(31)20-10-15-9-14(1-4-19(15)27-20)13-28-5-7-32-8-6-28/h1-4,9-12,27,30H,5-8,13,25H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SRC by time-resolved fluorescence or time-resolved fluorescence assay |
J Med Chem 59: 10586-10600 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01156
BindingDB Entry DOI: 10.7270/Q2ZS2ZGV |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074
(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 6x-His-tagged human Src kinase domain (T250 to L536 residues) expressed in Sf9 cells incubated for 2 hrs in presence of biotinylated cd... |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074

(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074

(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lille
Curated by ChEMBL
| Assay Description
Inhibition of Src |
Eur J Med Chem 45: 5678-84 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.022
BindingDB Entry DOI: 10.7270/Q2B27Z30 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074

(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals
| Assay Description
Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... |
Chem Biol Drug Des 67: 46-57 (2006)
Article DOI: 10.1111/j.1747-0285.2005.00316.x
BindingDB Entry DOI: 10.7270/Q2M61HRQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50025052
(CHEMBL517256)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)nc(CCC#N)nc12 Show InChI InChI=1S/C26H27N6OP/c1-18-7-5-8-19(2)22(18)14-16-32-17-28-24-25(30-23(9-6-15-27)31-26(24)32)29-20-10-12-21(13-11-20)34(3,4)33/h5,7-8,10-14,16-17H,6,9H2,1-4H3,(H,29,30,31)/b16-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6471
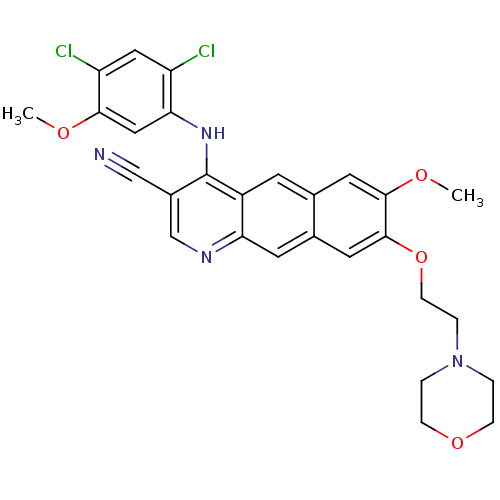
(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc(Nc2c(cnc3cc4cc(OCCN5CCOCC5)c(OC)cc4cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C28H26Cl2N4O4/c1-35-25-14-24(21(29)13-22(25)30)33-28-19(15-31)16-32-23-10-18-12-27(26(36-2)11-17(18)9-20(23)28)38-8-5-34-3-6-37-7-4-34/h9-14,16H,3-8H2,1-2H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of c-SRC |
Bioorg Med Chem 19: 2517-28 (2011)
Article DOI: 10.1016/j.bmc.2011.03.021
BindingDB Entry DOI: 10.7270/Q2DN45CD |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244569
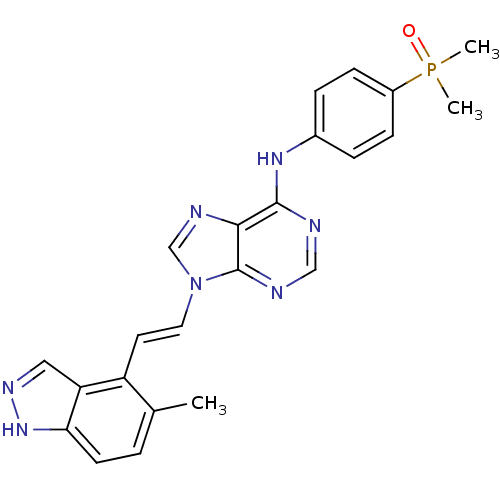
(AP24283 | CHEMBL510893 | N-(4-(dimethylphosphoryl)...)Show SMILES Cc1ccc2[nH]ncc2c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H22N7OP/c1-15-4-9-20-19(12-27-29-20)18(15)10-11-30-14-26-21-22(24-13-25-23(21)30)28-16-5-7-17(8-6-16)32(2,3)31/h4-14H,1-3H3,(H,27,29)(H,24,25,28)/b11-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6471

(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc(Nc2c(cnc3cc4cc(OCCN5CCOCC5)c(OC)cc4cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C28H26Cl2N4O4/c1-35-25-14-24(21(29)13-22(25)30)33-28-19(15-31)16-32-23-10-18-12-27(26(36-2)11-17(18)9-20(23)28)38-8-5-34-3-6-37-7-4-34/h9-14,16H,3-8H2,1-2H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
J Med Chem 48: 5909-20 (2005)
Article DOI: 10.1021/jm050512u
BindingDB Entry DOI: 10.7270/Q2833Q6N |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50244664
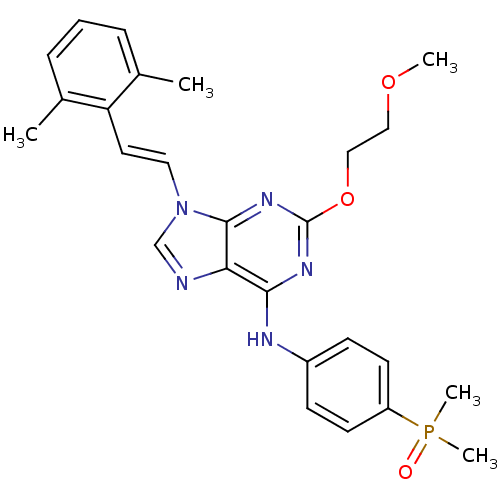
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES COCCOc1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C26H30N5O3P/c1-18-7-6-8-19(2)22(18)13-14-31-17-27-23-24(29-26(30-25(23)31)34-16-15-33-3)28-20-9-11-21(12-10-20)35(4,5)32/h6-14,17H,15-16H2,1-5H3,(H,28,29,30)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50025051
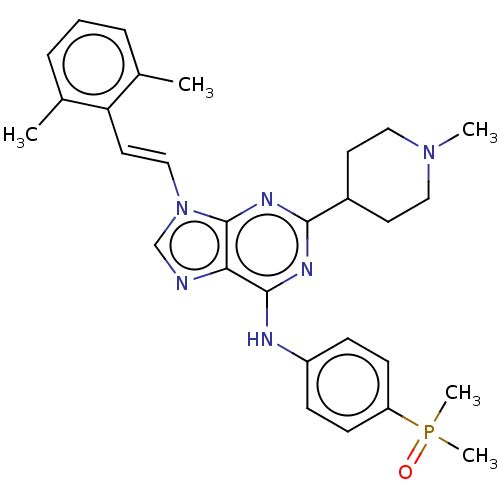
(CHEMBL461768)Show SMILES CN1CCC(CC1)c1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C29H35N6OP/c1-20-7-6-8-21(2)25(20)15-18-35-19-30-26-28(31-23-9-11-24(12-10-23)37(4,5)36)32-27(33-29(26)35)22-13-16-34(3)17-14-22/h6-12,15,18-19,22H,13-14,16-17H2,1-5H3,(H,31,32,33)/b18-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
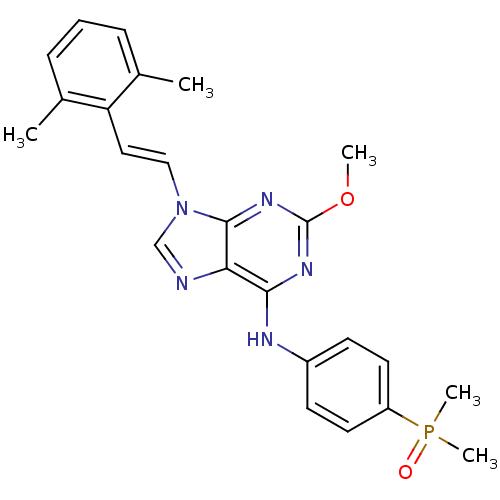
(Homo sapiens (Human)) | BDBM50244624
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES COc1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C24H26N5O2P/c1-16-7-6-8-17(2)20(16)13-14-29-15-25-21-22(27-24(31-3)28-23(21)29)26-18-9-11-19(12-10-18)32(4,5)30/h6-15H,1-5H3,(H,26,27,28)/b14-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
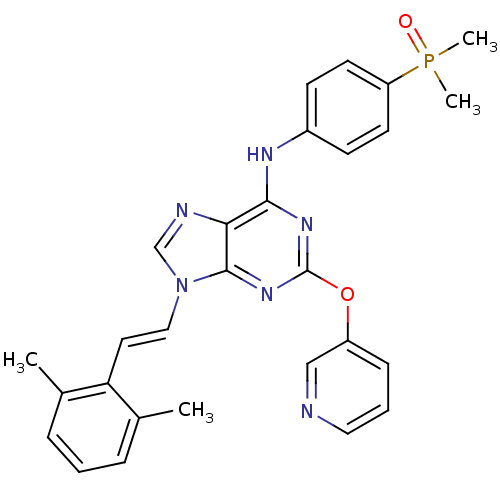
(Homo sapiens (Human)) | BDBM50244666
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)nc(Oc3cccnc3)nc12 Show InChI InChI=1S/C28H27N6O2P/c1-19-7-5-8-20(2)24(19)14-16-34-18-30-25-26(31-21-10-12-23(13-11-21)37(3,4)35)32-28(33-27(25)34)36-22-9-6-15-29-17-22/h5-18H,1-4H3,(H,31,32,33)/b16-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
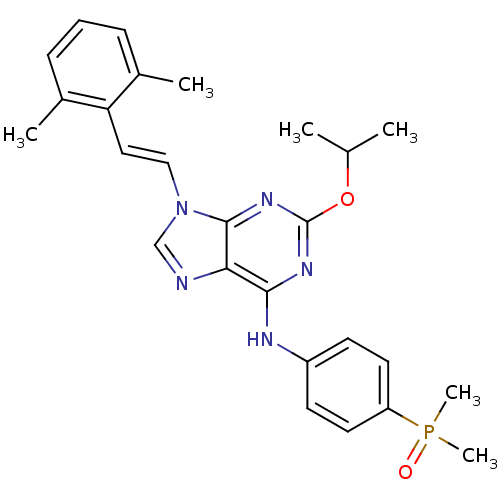
(Homo sapiens (Human)) | BDBM50244663
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES CC(C)Oc1nc(Nc2ccc(cc2)P(C)(C)=O)c2ncn(\C=C\c3c(C)cccc3C)c2n1 Show InChI InChI=1S/C26H30N5O2P/c1-17(2)33-26-29-24(28-20-10-12-21(13-11-20)34(5,6)32)23-25(30-26)31(16-27-23)15-14-22-18(3)8-7-9-19(22)4/h7-17H,1-6H3,(H,28,29,30)/b15-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
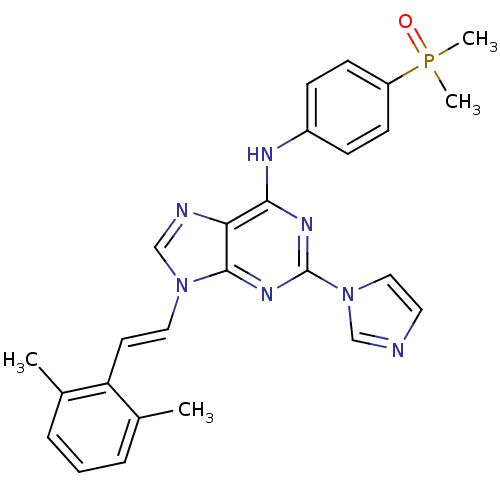
(Homo sapiens (Human)) | BDBM50244623
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)nc(nc12)-n1ccnc1 Show InChI InChI=1S/C26H26N7OP/c1-18-6-5-7-19(2)22(18)12-14-32-17-28-23-24(29-20-8-10-21(11-9-20)35(3,4)34)30-26(31-25(23)32)33-15-13-27-16-33/h5-17H,1-4H3,(H,29,30,31)/b14-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src kinase by TR-FRET assay |
Bioorg Med Chem Lett 18: 4907-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.042
BindingDB Entry DOI: 10.7270/Q2H41R78 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6476
(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc(Nc2c(cnc3cc4cc(OCCN5CCOCC5)c(OC)cc4cc23)C#N)c(Cl)cc1F Show InChI InChI=1S/C28H26ClFN4O4/c1-35-25-14-24(21(29)13-22(25)30)33-28-19(15-31)16-32-23-10-18-12-27(26(36-2)11-17(18)9-20(23)28)38-8-5-34-3-6-37-7-4-34/h9-14,16H,3-8H2,1-2H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
J Med Chem 48: 5909-20 (2005)
Article DOI: 10.1021/jm050512u
BindingDB Entry DOI: 10.7270/Q2833Q6N |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50252727
((2'Z-3'E)-6-Bromoindirubin-3'-(O-{2-[N-methyl,N-(2...)Show SMILES CN(CCO\N=C1\C(\Nc2ccccc\12)=C1\C(=O)Nc2cc(Br)ccc12)CC(O)CO Show InChI InChI=1S/C22H23BrN4O4/c1-27(11-14(29)12-28)8-9-31-26-20-16-4-2-3-5-17(16)24-21(20)19-15-7-6-13(23)10-18(15)25-22(19)30/h2-7,10,14,24,28-29H,8-9,11-12H2,1H3,(H,25,30)/b21-19-,26-20+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beckman Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Src (unknown origin) after 120 mins in presence of [33P]ATP |
J Nat Prod 79: 2464-2471 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00285
BindingDB Entry DOI: 10.7270/Q2GF0WZR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50252729
((2''Z-3''E)-6-Bromoindirubin-3''-(O-{2-[4-(2-hydro...)Show SMILES Cl.Cl.Brc1ccc2\C(C(=O)Nc2c1)=C1\Nc2ccccc2\C\1=N/OCCN1CCNCC1 Show InChI InChI=1S/C24H26BrN5O3/c25-16-5-6-17-20(15-16)27-24(32)21(17)23-22(18-3-1-2-4-19(18)26-23)28-33-14-12-30-9-7-29(8-10-30)11-13-31/h1-6,15,26,31H,7-14H2,(H,27,32)/b23-21-,28-22+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beckman Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Src (unknown origin) after 120 mins in presence of [33P]ATP |
J Nat Prod 79: 2464-2471 (2016)
Article DOI: 10.1021/acs.jnatprod.6b00285
BindingDB Entry DOI: 10.7270/Q2GF0WZR |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142887
(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP)
Curated by ChEMBL
| Assay Description
Inhibition of Src (unknown origin) using Src-family kinase bisamide rhodamine 110 peptide substrate after 1 hr by fluorescence assay |
Eur J Med Chem 157: 503-526 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.023
BindingDB Entry DOI: 10.7270/Q2DF6TX4 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00302
BindingDB Entry DOI: 10.7270/Q20Z779V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
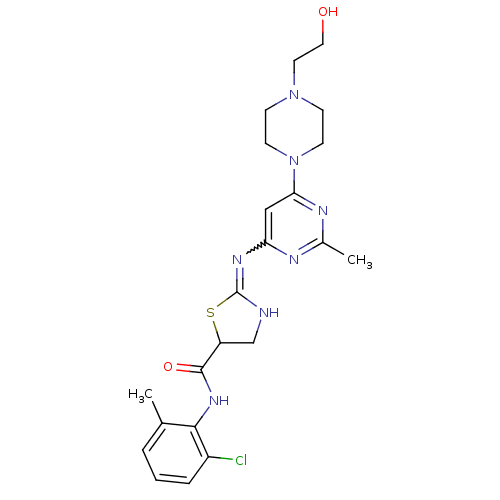
(Homo sapiens (Human)) | BDBM82130
(Dasatinib)Show SMILES Cc1nc(cc(n1)N1CCN(CCO)CC1)N=C1NCC(S1)C(=O)Nc1c(C)cccc1Cl |w:16.17| Show InChI InChI=1S/C22H28ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12,17,31H,6-11,13H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of src kinase |
J Med Chem 47: 6658-61 (2004)
Article DOI: 10.1021/jm049486a
BindingDB Entry DOI: 10.7270/Q2ZG6RRC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
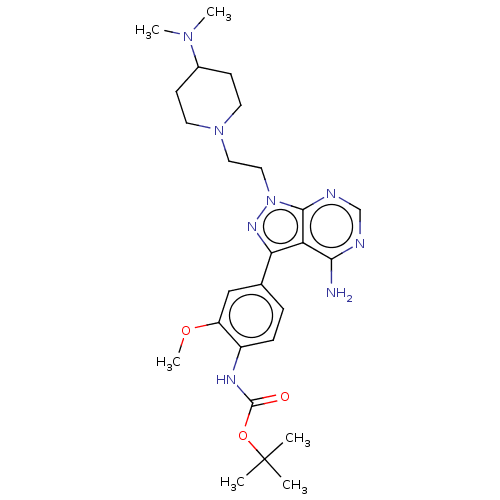
(Homo sapiens (Human)) | BDBM50184767
(CHEMBL3824089 | US10294227, Code 506)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N(C)C)c2ncnc(N)c12 Show InChI InChI=1S/C26H38N8O3/c1-26(2,3)37-25(35)30-19-8-7-17(15-20(19)36-6)22-21-23(27)28-16-29-24(21)34(31-22)14-13-33-11-9-18(10-12-33)32(4)5/h7-8,15-16,18H,9-14H2,1-6H3,(H,30,35)(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
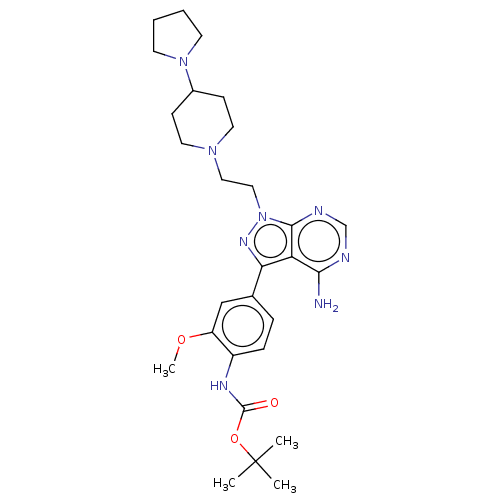
(Homo sapiens (Human)) | BDBM50184776
(CHEMBL3824233 | US10294227, Code 518)Show SMILES COc1cc(ccc1NC(=O)OC(C)(C)C)-c1nn(CCN2CCC(CC2)N2CCCC2)c2ncnc(N)c12 Show InChI InChI=1S/C28H40N8O3/c1-28(2,3)39-27(37)32-21-8-7-19(17-22(21)38-4)24-23-25(29)30-18-31-26(23)36(33-24)16-15-34-13-9-20(10-14-34)35-11-5-6-12-35/h7-8,17-18,20H,5-6,9-16H2,1-4H3,(H,32,37)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... |
J Med Chem 59: 4697-710 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00065
BindingDB Entry DOI: 10.7270/Q2B85B2D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
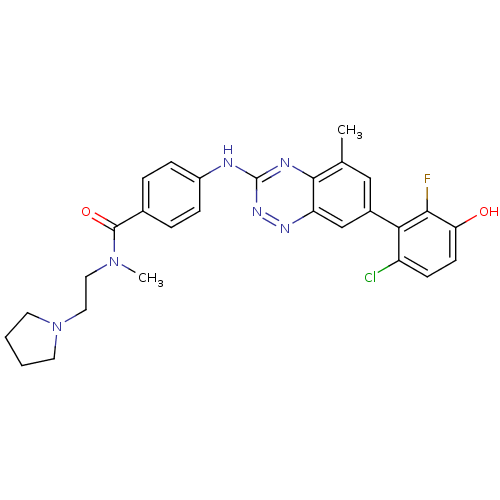
(Homo sapiens (Human)) | BDBM97975
(US8481536, 565)Show SMILES CN(CCN1CCCC1)C(=O)c1ccc(Nc2nnc3cc(cc(C)c3n2)-c2c(Cl)ccc(O)c2F)cc1 Show InChI InChI=1S/C28H28ClFN6O2/c1-17-15-19(24-21(29)9-10-23(37)25(24)30)16-22-26(17)32-28(34-33-22)31-20-7-5-18(6-8-20)27(38)35(2)13-14-36-11-3-4-12-36/h5-10,15-16,37H,3-4,11-14H2,1-2H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.542 | n/a | n/a | n/a | n/a | n/a | 25 |
TargeGen, Inc.
US Patent
| Assay Description
Testing of inhibition of kinases in vitro using luciferase-based assay from KinaseGlo, Promega Corp. |
US Patent US8481536 (2013)
BindingDB Entry DOI: 10.7270/Q2GB22PZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6475
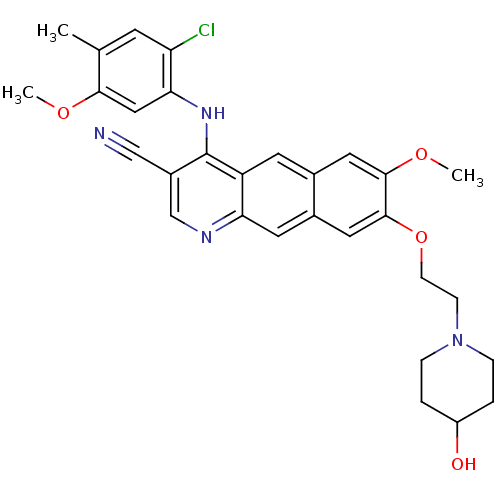
(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc(Nc2c(cnc3cc4cc(OCCN5CCC(O)CC5)c(OC)cc4cc23)C#N)c(Cl)cc1C Show InChI InChI=1S/C30H31ClN4O4/c1-18-10-24(31)26(15-27(18)37-2)34-30-21(16-32)17-33-25-12-20-14-29(28(38-3)13-19(20)11-23(25)30)39-9-8-35-6-4-22(36)5-7-35/h10-15,17,22,36H,4-9H2,1-3H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
J Med Chem 48: 5909-20 (2005)
Article DOI: 10.1021/jm050512u
BindingDB Entry DOI: 10.7270/Q2833Q6N |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6538
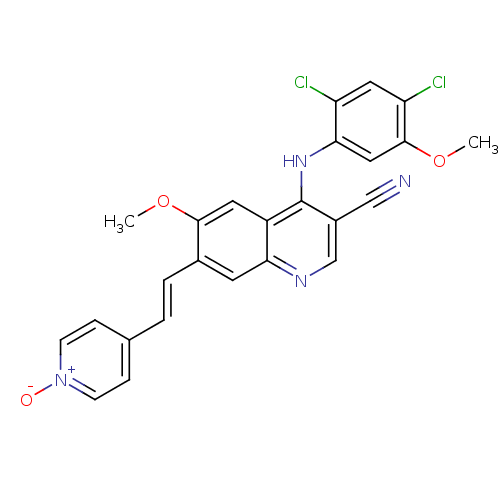
(4-[(E)-2-{3-cyano-4-[(2,4-dichloro-5-methoxyphenyl...)Show SMILES COc1cc(Nc2c(cnc3cc(\C=C\c4cc[n+]([O-])cc4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C25H18Cl2N4O3/c1-33-23-10-18-21(9-16(23)4-3-15-5-7-31(32)8-6-15)29-14-17(13-28)25(18)30-22-12-24(34-2)20(27)11-19(22)26/h3-12,14H,1-2H3,(H,29,30)/b4-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
| Assay Description
Kinase assays were performed using the europium/APC detection format (LANCE, Perkin Elmer). HTRF is based on the proximity of europium cryptate (dono... |
Bioorg Med Chem Lett 15: 1743-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.004
BindingDB Entry DOI: 10.7270/Q2VX0DRH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data