

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3444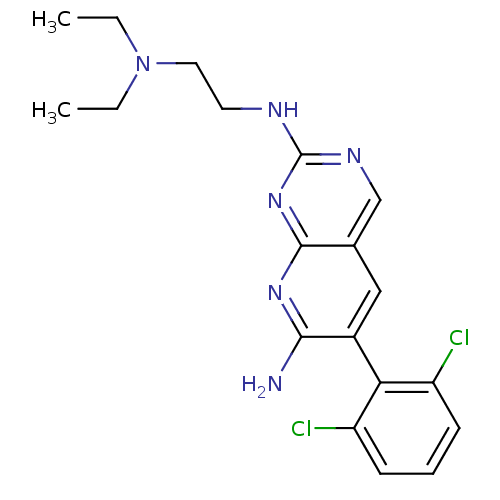 (6-(2,6-dichlorophenyl)-2-N-[2-(diethylamino)ethyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3449 (6-(2,6-dichlorophenyl)-2-N-[4-(diethylamino)butyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3454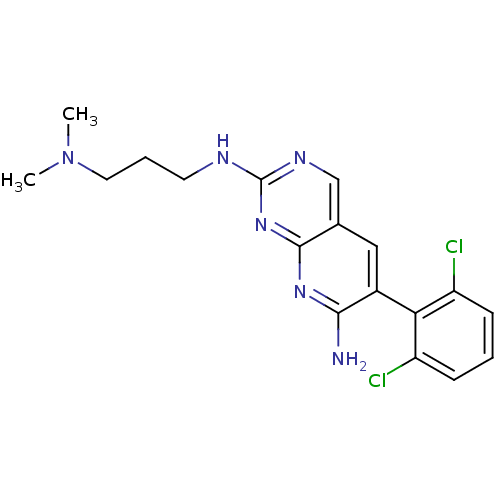 (6-(2,6-dichlorophenyl)-2-N-[3-(dimethylamino)propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3456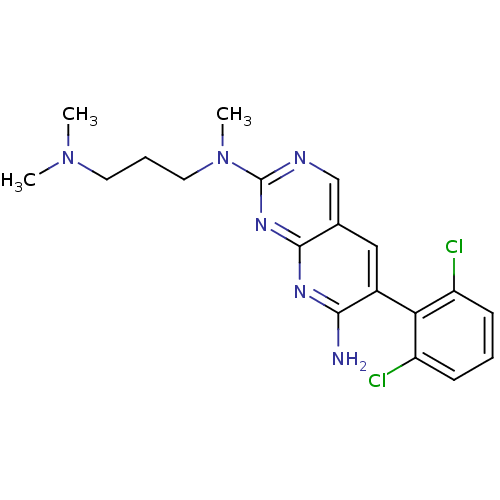 (6-(2,6-dichlorophenyl)-2-N-[3-(dimethylamino)propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3458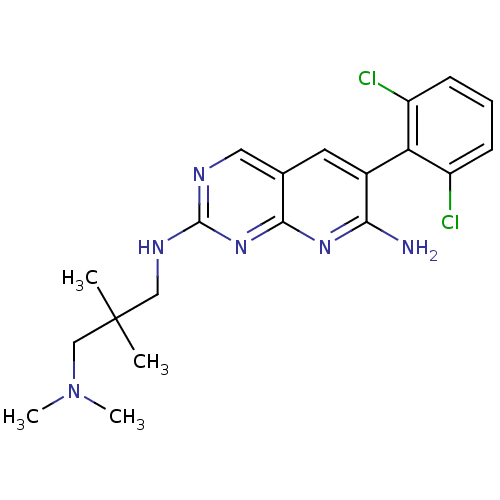 (6-(2,6-dichlorophenyl)-2-N-{2-[(dimethylamino)meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3460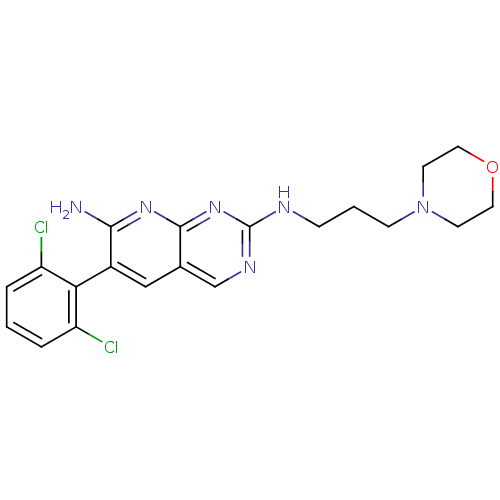 (6-(2,6-dichlorophenyl)-2-N-[3-(morpholin-4-yl)prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50303586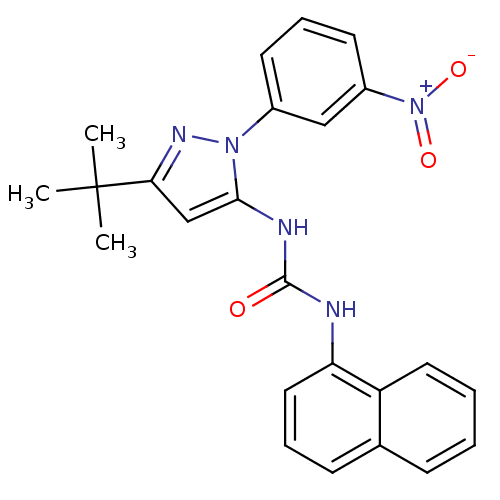 (1-(3-tert-Butyl-1-(3-nitrophenyl)-1H-pyrazol-5-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society | Assay Description Tyrosine kinase inhibition assay using wild type cSrc measured by a fluorescence-labeled approach. | Nat Chem Biol 5: 394-6 (2009) Article DOI: 10.1038/nchembio.162 BindingDB Entry DOI: 10.7270/Q20V8B46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3466 (6-(2,6-dichlorophenyl)-2-N-[4-(4-methylpiperazin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4495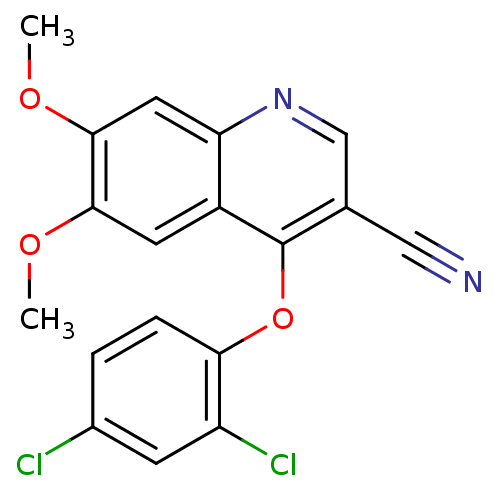 (4-(2,4-Dichlorophenoxy)-6,7-dimethoxy-3-quinolinec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 822-33 (2001) Article DOI: 10.1021/jm000420z BindingDB Entry DOI: 10.7270/Q2Z31WVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4497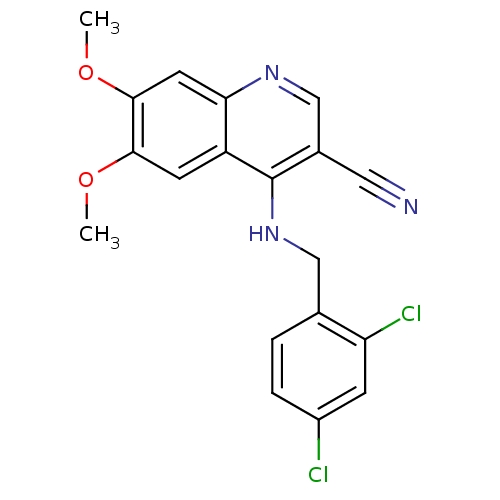 (4-[(2,4-Dichlorophenylamino)methyl]-6,7-dimethoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 822-33 (2001) Article DOI: 10.1021/jm000420z BindingDB Entry DOI: 10.7270/Q2Z31WVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4498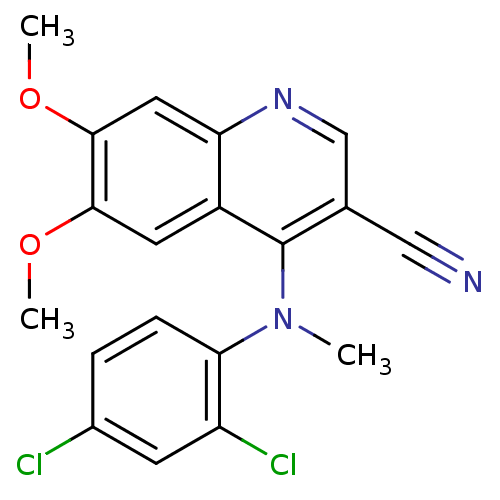 (4-(2,4-Dichlorophenylmethylamino)-6,7-dimethoxy-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 822-33 (2001) Article DOI: 10.1021/jm000420z BindingDB Entry DOI: 10.7270/Q2Z31WVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4503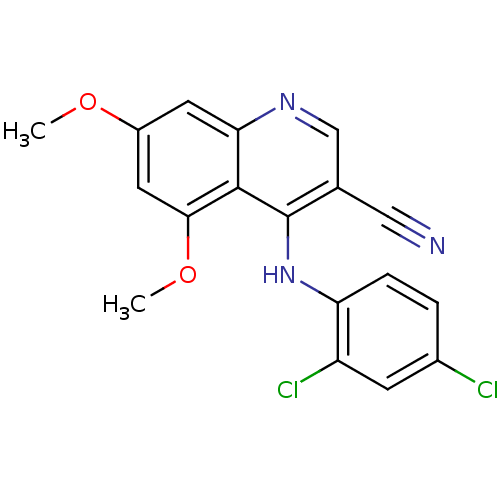 (3-quinolinecarbonitrile deriv. 2g | 4-[(2,4-Dichlo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 822-33 (2001) Article DOI: 10.1021/jm000420z BindingDB Entry DOI: 10.7270/Q2Z31WVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50115216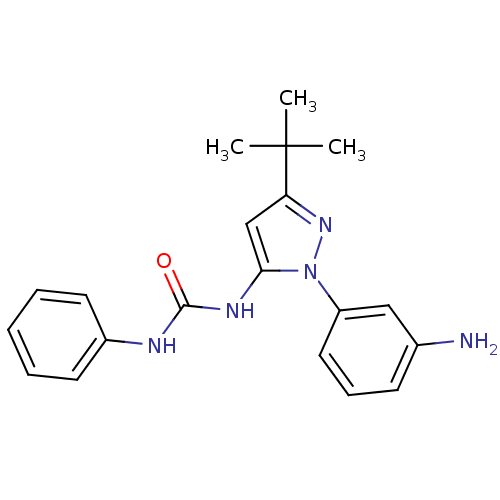 (1-(1-(3-Aminophenyl)-3-tert-butyl-1H-pyrazol-5-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society | Assay Description Tyrosine kinase inhibition assay using wild type cSrc measured by a fluorescence-labeled approach. | Nat Chem Biol 5: 394-6 (2009) Article DOI: 10.1038/nchembio.162 BindingDB Entry DOI: 10.7270/Q20V8B46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3462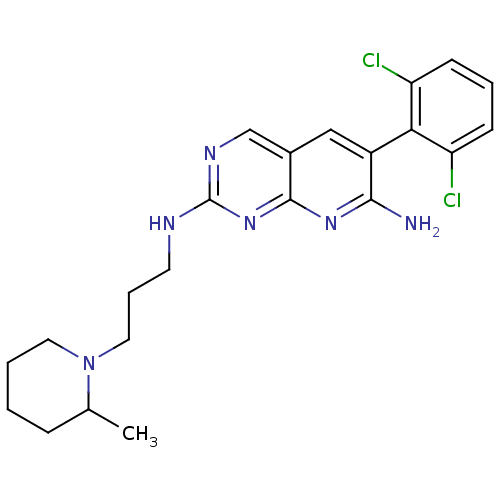 (6-(2,6-dichlorophenyl)-2-N-[3-(2-methylpiperidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 44: 1915-26 (2001) Article DOI: 10.1021/jm0004291 BindingDB Entry DOI: 10.7270/Q29K48DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||