 Found 3101 hits of ic50 for UniProtKB: P00742
Found 3101 hits of ic50 for UniProtKB: P00742 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266775

((2R,4S)-4-(2-Chlorophenyl)-N1-(4-chlorophenyl)-4-h...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1Cl |r| Show InChI InChI=1S/C28H23Cl2N5O4/c29-18-8-10-19(11-9-18)32-27(38)35-17-28(39,21-5-1-2-6-22(21)30)15-23(35)26(37)33-24-13-12-20(16-31-24)34-14-4-3-7-25(34)36/h1-14,16,23,39H,15,17H2,(H,32,38)(H,31,33,37)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266920

((2R,4R)-N1-(4-Chlorophenyl)-4-ethoxy-4-ethyl-N2-(2...)Show SMILES CCO[C@]1(CC)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C27H28ClFN4O4/c1-3-27(37-4-2)16-23(33(17-27)26(36)30-19-10-8-18(28)9-11-19)25(35)31-22-13-12-20(15-21(22)29)32-14-6-5-7-24(32)34/h5-15,23H,3-4,16-17H2,1-2H3,(H,30,36)(H,31,35)/t23-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266923
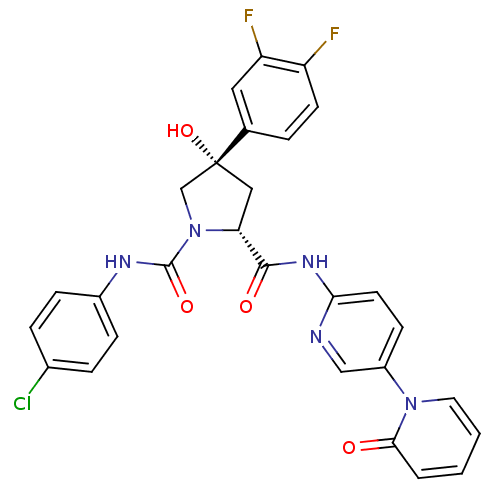
((2R,4S)-N1-(4-Chlorophenyl)-4-(3,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-18-5-7-19(8-6-18)33-27(39)36-16-28(40,17-4-10-21(30)22(31)13-17)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266921
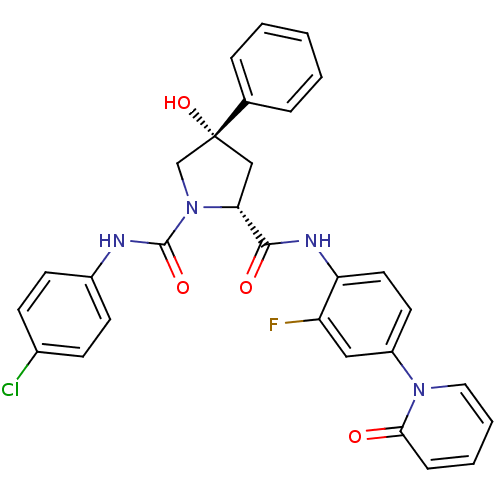
((2R,4S)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(2-oxop...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)c1ccccc1 |r| Show InChI InChI=1S/C29H24ClFN4O4/c30-20-9-11-21(12-10-20)32-28(38)35-18-29(39,19-6-2-1-3-7-19)17-25(35)27(37)33-24-14-13-22(16-23(24)31)34-15-5-4-8-26(34)36/h1-16,25,39H,17-18H2,(H,32,38)(H,33,37)/t25-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266924
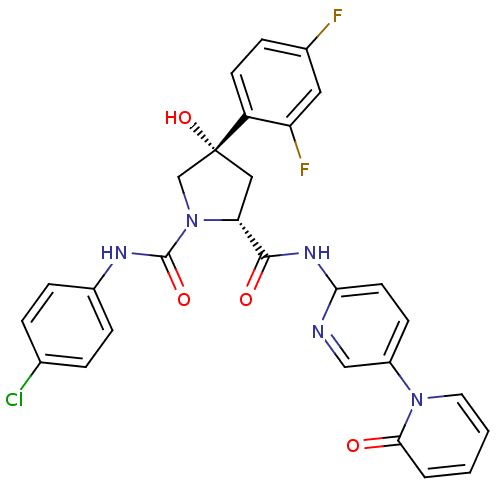
((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266924

((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266773

((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1ccccc1[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-6-2-3-7-23(19)29(39)16-24(35(18-29)28(38)32-21-11-9-20(30)10-12-21)27(37)33-25-14-13-22(17-31-25)34-15-5-4-8-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328697

((R)-5-guanidino-N-(2-((S)-5-guanidino-1-oxo-1-(thi...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-c1nccs1 Show InChI InChI=1S/C24H36N10O5S2/c25-23(26)30-10-4-8-17(20(36)22-29-12-13-40-22)33-19(35)14-32-21(37)18(9-5-11-31-24(27)28)34-41(38,39)15-16-6-2-1-3-7-16/h1-3,6-7,12-13,17-18,34H,4-5,8-11,14-15H2,(H,32,37)(H,33,35)(H4,25,26,30)(H4,27,28,31)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266922
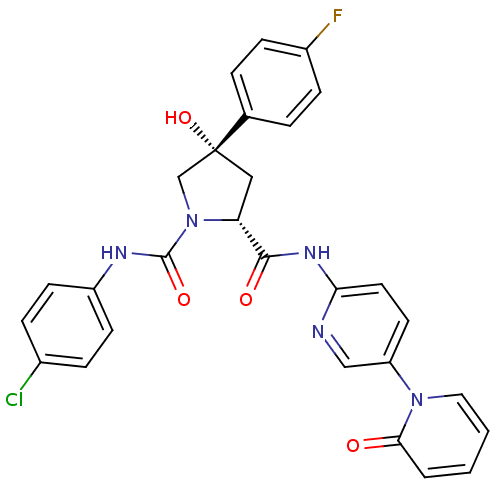
((2R,4S)-N1-(4-Chlorophenyl)-4-(4-fluorophenyl)-4-h...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C28H23ClFN5O4/c29-19-6-10-21(11-7-19)32-27(38)35-17-28(39,18-4-8-20(30)9-5-18)15-23(35)26(37)33-24-13-12-22(16-31-24)34-14-2-1-3-25(34)36/h1-14,16,23,39H,15,17H2,(H,32,38)(H,31,33,37)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328726
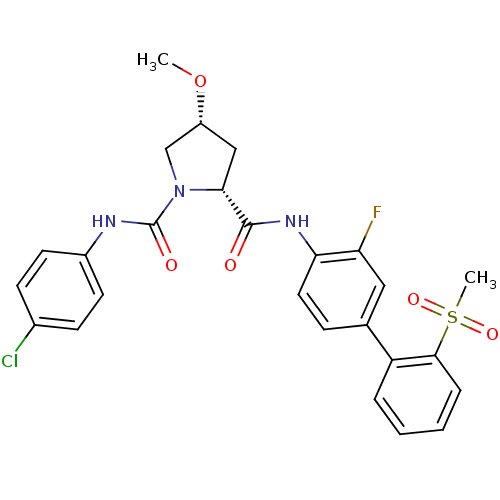
((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C26H25ClFN3O5S/c1-36-19-14-23(31(15-19)26(33)29-18-10-8-17(27)9-11-18)25(32)30-22-12-7-16(13-21(22)28)20-5-3-4-6-24(20)37(2,34)35/h3-13,19,23H,14-15H2,1-2H3,(H,29,33)(H,30,32)/t19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328726

((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C26H25ClFN3O5S/c1-36-19-14-23(31(15-19)26(33)29-18-10-8-17(27)9-11-18)25(32)30-22-12-7-16(13-21(22)28)20-5-3-4-6-24(20)37(2,34)35/h3-13,19,23H,14-15H2,1-2H3,(H,29,33)(H,30,32)/t19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266743
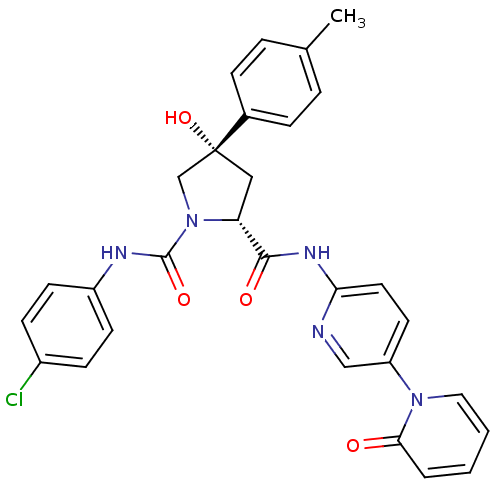
((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1ccc(cc1)[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-5-7-20(8-6-19)29(39)16-24(35(18-29)28(38)32-22-11-9-21(30)10-12-22)27(37)33-25-14-13-23(17-31-25)34-15-3-2-4-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266893
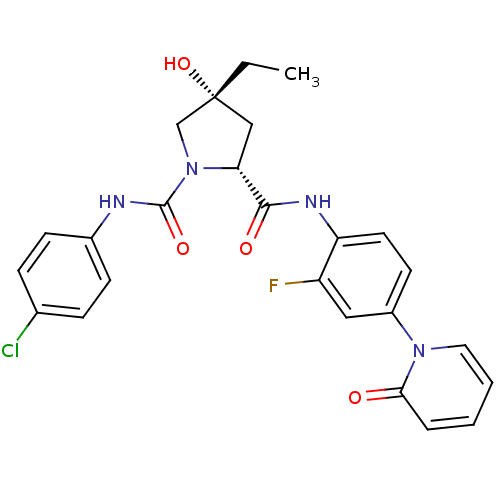
((2R,4R)-N1-(4-Chlorophenyl)-4-ethyl-N2-(2-fluoro-4...)Show SMILES CC[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C25H24ClFN4O4/c1-2-25(35)14-21(31(15-25)24(34)28-17-8-6-16(26)7-9-17)23(33)29-20-11-10-18(13-19(20)27)30-12-4-3-5-22(30)32/h3-13,21,35H,2,14-15H2,1H3,(H,28,34)(H,29,33)/t21-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50103666
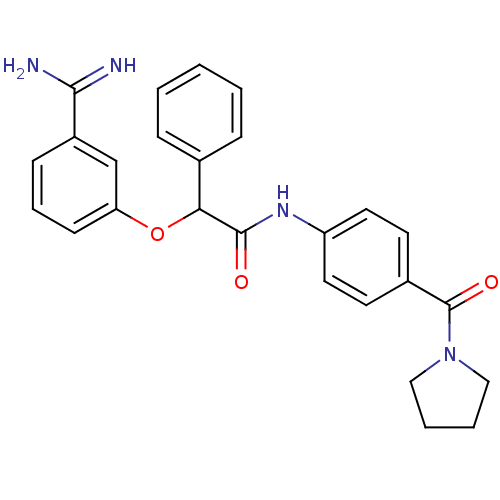
(2-(3-Carbamimidoyl-phenoxy)-2-phenyl-N-[4-(pyrroli...)Show SMILES NC(=N)c1cccc(OC(C(=O)Nc2ccc(cc2)C(=O)N2CCCC2)c2ccccc2)c1 Show InChI InChI=1S/C26H26N4O3/c27-24(28)20-9-6-10-22(17-20)33-23(18-7-2-1-3-8-18)25(31)29-21-13-11-19(12-14-21)26(32)30-15-4-5-16-30/h1-3,6-14,17,23H,4-5,15-16H2,(H3,27,28)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X |
Bioorg Med Chem Lett 11: 2279-82 (2001)
BindingDB Entry DOI: 10.7270/Q2SQ8ZN4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113591

((E)-3-(3-Carbamimidoyl-phenyl)-2-fluoro-but-2-enoi...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)c1cccc(c1)C(N)=N Show InChI InChI=1S/C23H20F2N4O3S/c1-13(14-5-4-6-16(11-14)22(26)27)21(25)23(30)29-19-10-9-15(12-18(19)24)17-7-2-3-8-20(17)33(28,31)32/h2-12H,1H3,(H3,26,27)(H,29,30)(H2,28,31,32)/b21-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against factor Xa |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113591

((E)-3-(3-Carbamimidoyl-phenyl)-2-fluoro-but-2-enoi...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)c1cccc(c1)C(N)=N Show InChI InChI=1S/C23H20F2N4O3S/c1-13(14-5-4-6-16(11-14)22(26)27)21(25)23(30)29-19-10-9-15(12-18(19)24)17-7-2-3-8-20(17)33(28,31)32/h2-12H,1H3,(H3,26,27)(H,29,30)(H2,28,31,32)/b21-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration to coagulation factor X |
Bioorg Med Chem Lett 13: 297-300 (2002)
BindingDB Entry DOI: 10.7270/Q2XD112T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443857
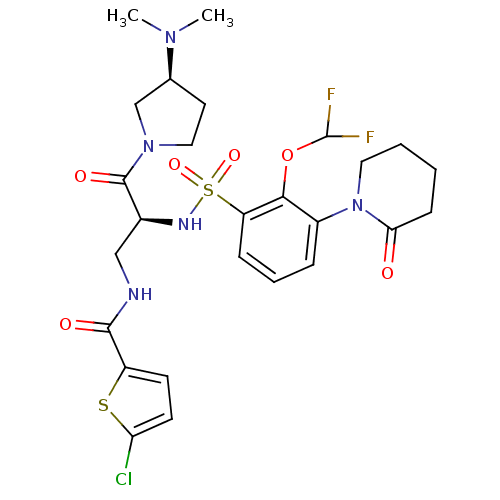
(CHEMBL3091501)Show SMILES CN(C)[C@H]1CCN(C1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCCC2=O)c1OC(F)F |r| Show InChI InChI=1S/C26H32ClF2N5O6S2/c1-32(2)16-11-13-33(15-16)25(37)17(14-30-24(36)19-9-10-21(27)41-19)31-42(38,39)20-7-5-6-18(23(20)40-26(28)29)34-12-4-3-8-22(34)35/h5-7,9-10,16-17,26,31H,3-4,8,11-15H2,1-2H3,(H,30,36)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142090
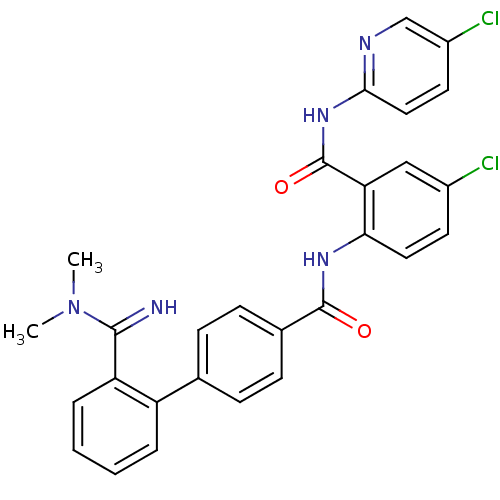
(2'-(N,N-Dimethyl-carbamimidoyl)-biphenyl-4-carboxy...)Show SMILES CN(C)C(=N)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-35(2)26(31)22-6-4-3-5-21(22)17-7-9-18(10-8-17)27(36)33-24-13-11-19(29)15-23(24)28(37)34-25-14-12-20(30)16-32-25/h3-16,31H,1-2H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142163

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(2-imino-o...)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(CN3CCOC3=N)cc2)nc1 Show InChI InChI=1S/C23H19Cl2N5O3/c24-16-5-7-19(18(11-16)22(32)29-20-8-6-17(25)12-27-20)28-21(31)15-3-1-14(2-4-15)13-30-9-10-33-23(30)26/h1-8,11-12,26H,9-10,13H2,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266919
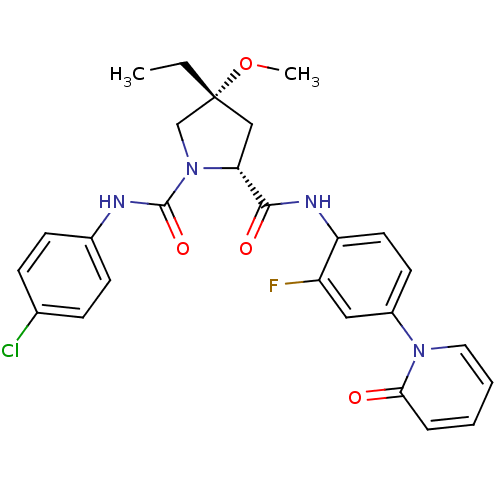
((2R,4R)-N1-(4-Chlorophenyl)-4-ethyl-N2-(2-fluoro-4...)Show SMILES CC[C@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)OC |r| Show InChI InChI=1S/C26H26ClFN4O4/c1-3-26(36-2)15-22(32(16-26)25(35)29-18-9-7-17(27)8-10-18)24(34)30-21-12-11-19(14-20(21)28)31-13-5-4-6-23(31)33/h4-14,22H,3,15-16H2,1-2H3,(H,29,35)(H,30,34)/t22-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142161
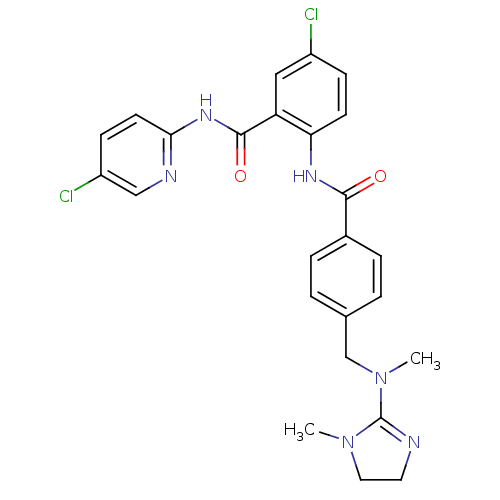
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-(4-{[methyl-(...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C1=NCCN1C |t:32| Show InChI InChI=1S/C25H24Cl2N6O2/c1-32-12-11-28-25(32)33(2)15-16-3-5-17(6-4-16)23(34)30-21-9-7-18(26)13-20(21)24(35)31-22-10-8-19(27)14-29-22/h3-10,13-14H,11-12,15H2,1-2H3,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266891

((2R,4R)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(2-oxop...)Show SMILES CO[C@]1(C)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C25H24ClFN4O4/c1-25(35-2)14-21(31(15-25)24(34)28-17-8-6-16(26)7-9-17)23(33)29-20-11-10-18(13-19(20)27)30-12-4-3-5-22(30)32/h3-13,21H,14-15H2,1-2H3,(H,28,34)(H,29,33)/t21-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266770

((2R,4R)-N~1~-(4-CHLOROPHENYL)-N~2~-[2-FLUORO-4-(2-...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C24H22ClFN4O4/c1-34-18-13-21(30(14-18)24(33)27-16-7-5-15(25)6-8-16)23(32)28-20-10-9-17(12-19(20)26)29-11-3-2-4-22(29)31/h2-12,18,21H,13-14H2,1H3,(H,27,33)(H,28,32)/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266774

((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C29H23ClF3N5O4/c30-18-8-10-19(11-9-18)35-27(41)38-17-28(42,21-5-1-2-6-22(21)29(31,32)33)15-23(38)26(40)36-24-13-12-20(16-34-24)37-14-4-3-7-25(37)39/h1-14,16,23,42H,15,17H2,(H,35,41)(H,34,36,40)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328728
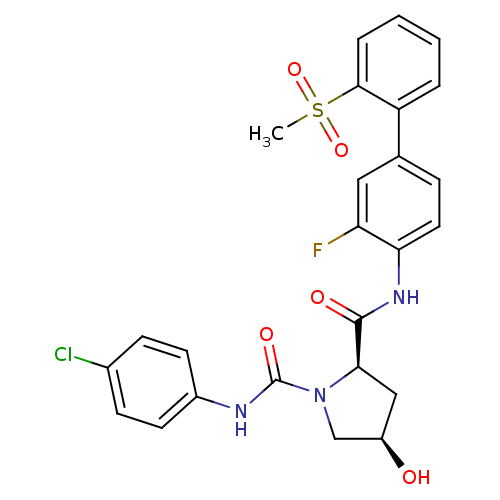
((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2C[C@@H](O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H23ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,18,22,31H,13-14H2,1H3,(H,28,33)(H,29,32)/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358999

(CHEMBL1923892)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1cc(cc(Cl)c1CCC(=O)Nc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C26H26Cl2N4O4S/c1-14(2)32-10-9-20-22(13-32)37-25(31-20)24(34)30-21-12-15(26(35)36)11-19(28)18(21)7-8-23(33)29-17-5-3-16(27)4-6-17/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,29,33)(H,30,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328728

((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2C[C@@H](O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H23ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,18,22,31H,13-14H2,1H3,(H,28,33)(H,29,32)/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50559550
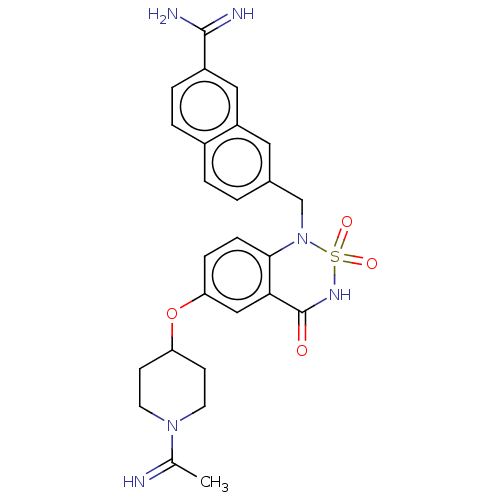
(CHEMBL4746164)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2N(Cc3ccc4ccc(cc4c3)C(N)=N)S(=O)(=O)NC(=O)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human factor Xa using S-2238 as substrate by chromogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00460
BindingDB Entry DOI: 10.7270/Q21Z483R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249166

(2-(azetidin-1-yl)-N-(4-chloro-2-(5-chloropyridin-2...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(c1)N1CCC1 Show InChI InChI=1S/C25H24Cl2N6O2/c1-32(2)23(28)15-4-7-18(21(12-15)33-10-3-11-33)24(34)30-20-8-5-16(26)13-19(20)25(35)31-22-9-6-17(27)14-29-22/h4-9,12-14,28H,3,10-11H2,1-2H3,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7842

(BAY 59-7939 Analog 17 | US8822458, 46)Show SMILES Brc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18BrN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG
| Assay Description
The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... |
J Med Chem 48: 5900-8 (2005)
Article DOI: 10.1021/jm050101d
BindingDB Entry DOI: 10.7270/Q2J38QR6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142111
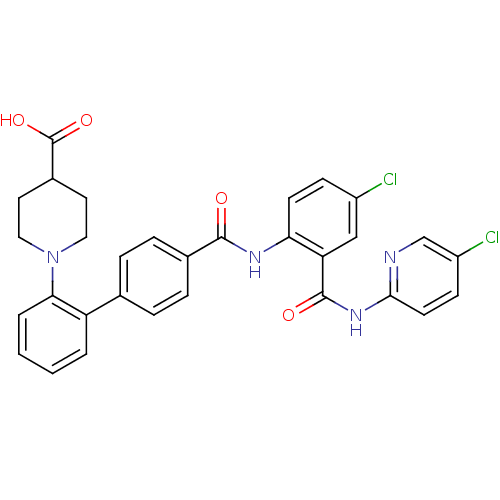
(1-{4'-[4-Chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H26Cl2N4O4/c32-22-9-11-26(25(17-22)30(39)36-28-12-10-23(33)18-34-28)35-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)37-15-13-21(14-16-37)31(40)41/h1-12,17-18,21H,13-16H2,(H,35,38)(H,40,41)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35767
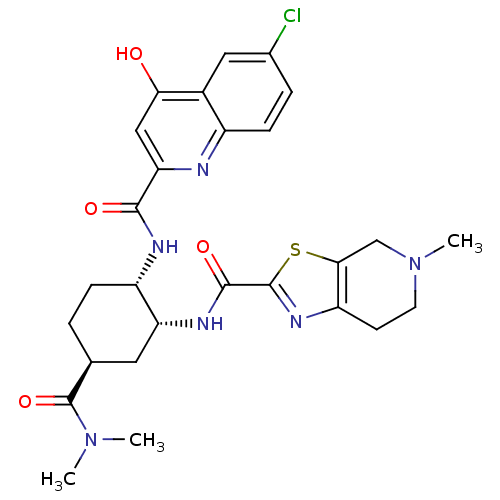
(cis-1,2-diaminocyclohexane derivative, 5h)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc(O)c3cc(Cl)ccc3n2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C27H31ClN6O4S/c1-33(2)27(38)14-4-6-18(30-24(36)21-12-22(35)16-11-15(28)5-7-17(16)29-21)20(10-14)31-25(37)26-32-19-8-9-34(3)13-23(19)39-26/h5,7,11-12,14,18,20H,4,6,8-10,13H2,1-3H3,(H,29,35)(H,30,36)(H,31,37)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Daiichi Sankyo Co, Ltd
| Assay Description
The in vitro anti-fXa activity was measured by using a chromogenic substrate S-2222 and human fXa. Aqueous DMSO or test compounds in aqueous DMSO and... |
Bioorg Med Chem 17: 8221-33 (2009)
Article DOI: 10.1016/j.bmc.2009.10.024
BindingDB Entry DOI: 10.7270/Q2CC0Z1V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443853
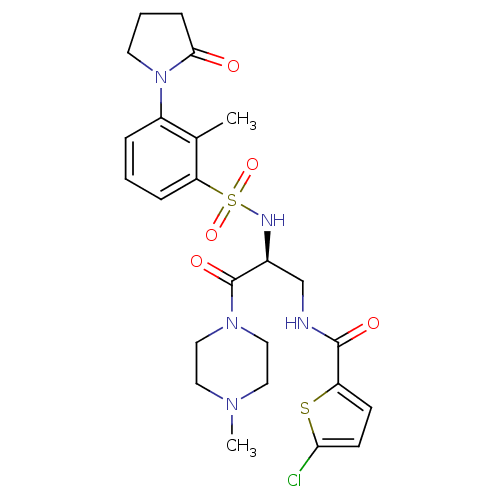
(CHEMBL3091519 | US20230391761, Reference 1)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| Show InChI InChI=1S/C24H30ClN5O5S2/c1-16-18(30-10-4-7-22(30)31)5-3-6-20(16)37(34,35)27-17(24(33)29-13-11-28(2)12-14-29)15-26-23(32)19-8-9-21(25)36-19/h3,5-6,8-9,17,27H,4,7,10-15H2,1-2H3,(H,26,32)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639340
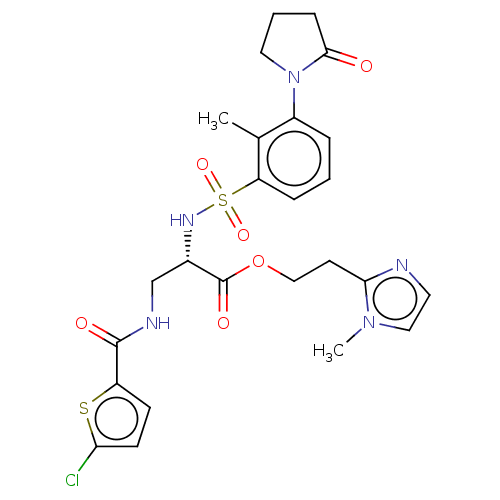
(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...)Show SMILES Cc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639343

(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50359000
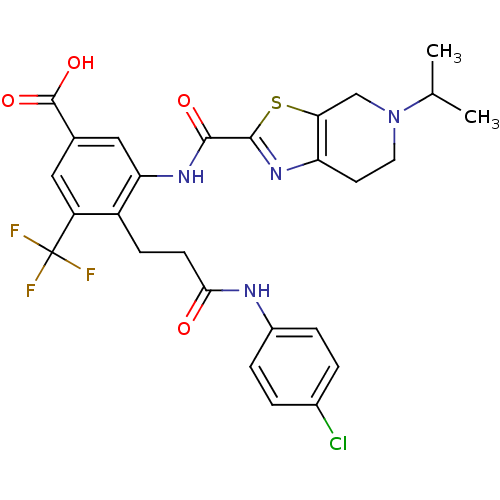
(CHEMBL1923893)Show SMILES CC(C)N1CCc2nc(sc2C1)C(=O)Nc1cc(cc(c1CCC(=O)Nc1ccc(Cl)cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C27H26ClF3N4O4S/c1-14(2)35-10-9-20-22(13-35)40-25(34-20)24(37)33-21-12-15(26(38)39)11-19(27(29,30)31)18(21)7-8-23(36)32-17-5-3-16(28)4-6-17/h3-6,11-12,14H,7-10,13H2,1-2H3,(H,32,36)(H,33,37)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 as substrate by spectrophotometry |
Bioorg Med Chem Lett 21: 7337-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.021
BindingDB Entry DOI: 10.7270/Q2V40VM5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639321

(Methyl 3-{[(5-chloro-2-thienyl)carbonyl]amino}-N-{...)Show SMILES COC(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249118
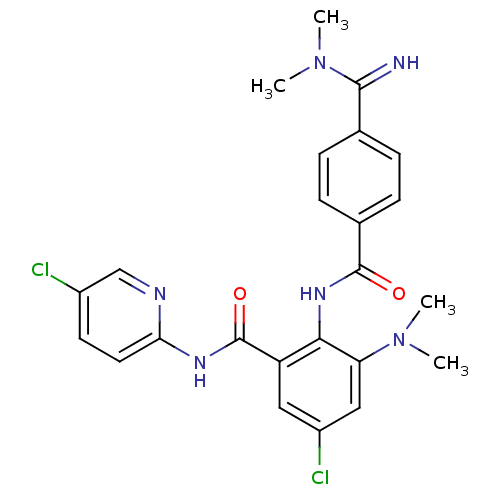
(5-chloro-N-(5-chloropyridin-2-yl)-3-(dimethylamino...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1c(cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)N(C)C Show InChI InChI=1S/C24H24Cl2N6O2/c1-31(2)19-12-17(26)11-18(24(34)29-20-10-9-16(25)13-28-20)21(19)30-23(33)15-7-5-14(6-8-15)22(27)32(3)4/h5-13,27H,1-4H3,(H,30,33)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249117
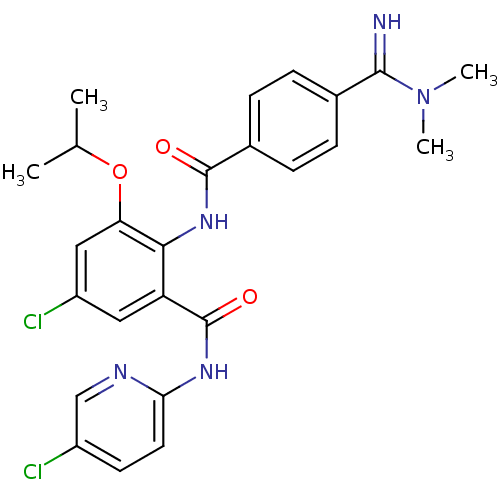
(5-chloro-N-(5-chloropyridin-2-yl)-2-(4-(N,N-dimeth...)Show SMILES CC(C)Oc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)C(=N)N(C)C Show InChI InChI=1S/C25H25Cl2N5O3/c1-14(2)35-20-12-18(27)11-19(25(34)30-21-10-9-17(26)13-29-21)22(20)31-24(33)16-7-5-15(6-8-16)23(28)32(3)4/h5-14,28H,1-4H3,(H,31,33)(H,29,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266742
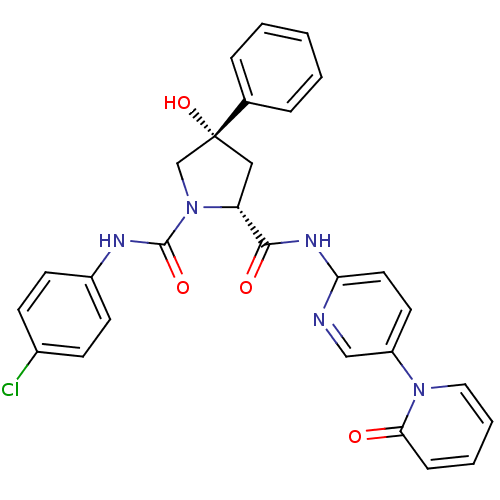
((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1 |r| Show InChI InChI=1S/C28H24ClN5O4/c29-20-9-11-21(12-10-20)31-27(37)34-18-28(38,19-6-2-1-3-7-19)16-23(34)26(36)32-24-14-13-22(17-30-24)33-15-5-4-8-25(33)35/h1-15,17,23,38H,16,18H2,(H,31,37)(H,30,32,36)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266892
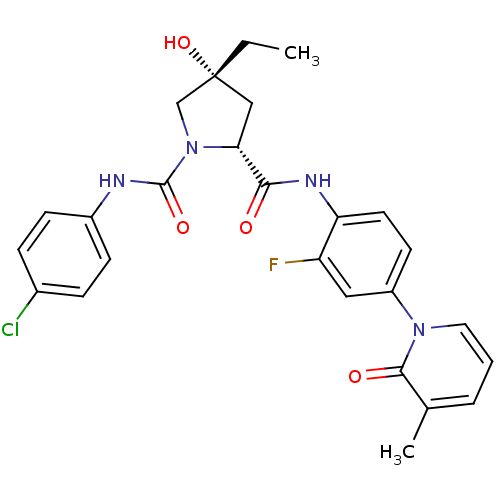
((2R,4R)-N1-(4-Chlorophenyl)-4-ethyl-N2-(2-fluoro-4...)Show SMILES CC[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1cccc(C)c1=O |r| Show InChI InChI=1S/C26H26ClFN4O4/c1-3-26(36)14-22(32(15-26)25(35)29-18-8-6-17(27)7-9-18)23(33)30-21-11-10-19(13-20(21)28)31-12-4-5-16(2)24(31)34/h4-13,22,36H,3,14-15H2,1-2H3,(H,29,35)(H,30,33)/t22-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113590
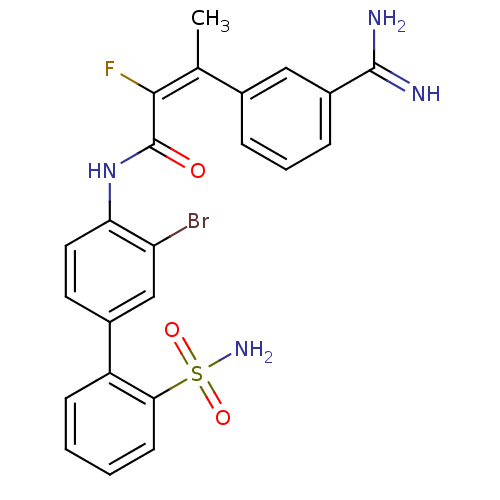
((E)-3-(3-Carbamimidoyl-phenyl)-2-fluoro-but-2-enoi...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Br)-c1ccccc1S(N)(=O)=O)c1cccc(c1)C(N)=N Show InChI InChI=1S/C23H20BrFN4O3S/c1-13(14-5-4-6-16(11-14)22(26)27)21(25)23(30)29-19-10-9-15(12-18(19)24)17-7-2-3-8-20(17)33(28,31)32/h2-12H,1H3,(H3,26,27)(H,29,30)(H2,28,31,32)/b21-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration to coagulation factor X |
Bioorg Med Chem Lett 13: 297-300 (2002)
BindingDB Entry DOI: 10.7270/Q2XD112T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113586
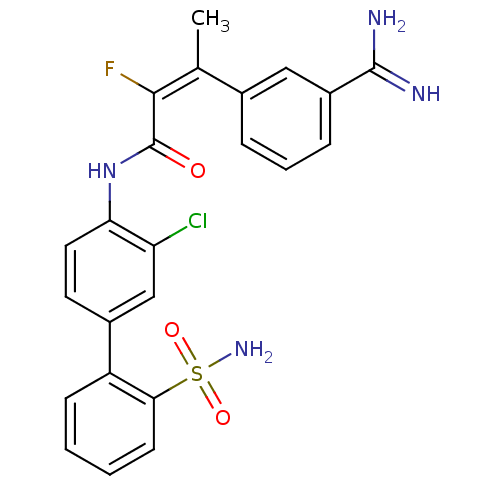
((E)-3-(3-Carbamimidoyl-phenyl)-2-fluoro-but-2-enoi...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Cl)-c1ccccc1S(N)(=O)=O)c1cccc(c1)C(N)=N Show InChI InChI=1S/C23H20ClFN4O3S/c1-13(14-5-4-6-16(11-14)22(26)27)21(25)23(30)29-19-10-9-15(12-18(19)24)17-7-2-3-8-20(17)33(28,31)32/h2-12H,1H3,(H3,26,27)(H,29,30)(H2,28,31,32)/b21-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration to coagulation factor X |
Bioorg Med Chem Lett 13: 297-300 (2002)
BindingDB Entry DOI: 10.7270/Q2XD112T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113590

((E)-3-(3-Carbamimidoyl-phenyl)-2-fluoro-but-2-enoi...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Br)-c1ccccc1S(N)(=O)=O)c1cccc(c1)C(N)=N Show InChI InChI=1S/C23H20BrFN4O3S/c1-13(14-5-4-6-16(11-14)22(26)27)21(25)23(30)29-19-10-9-15(12-18(19)24)17-7-2-3-8-20(17)33(28,31)32/h2-12H,1H3,(H3,26,27)(H,29,30)(H2,28,31,32)/b21-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against factor Xa |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113586

((E)-3-(3-Carbamimidoyl-phenyl)-2-fluoro-but-2-enoi...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Cl)-c1ccccc1S(N)(=O)=O)c1cccc(c1)C(N)=N Show InChI InChI=1S/C23H20ClFN4O3S/c1-13(14-5-4-6-16(11-14)22(26)27)21(25)23(30)29-19-10-9-15(12-18(19)24)17-7-2-3-8-20(17)33(28,31)32/h2-12H,1H3,(H3,26,27)(H,29,30)(H2,28,31,32)/b21-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against factor Xa |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124984
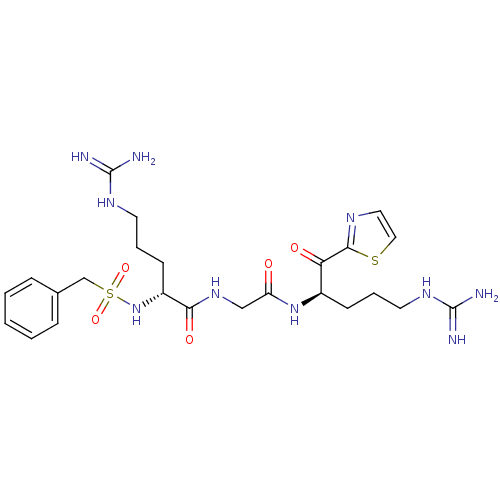
((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C24H36N10O5S2/c25-23(26)30-10-4-8-17(20(36)22-29-12-13-40-22)33-19(35)14-32-21(37)18(9-5-11-31-24(27)28)34-41(38,39)15-16-6-2-1-3-7-16/h1-3,6-7,12-13,17-18,34H,4-5,8-11,14-15H2,(H,32,37)(H,33,35)(H4,25,26,30)(H4,27,28,31)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443858
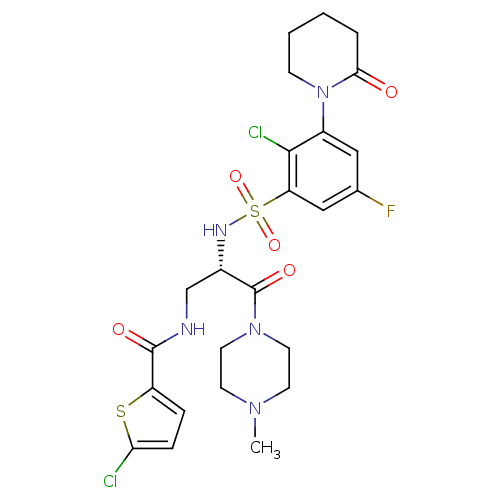
(CHEMBL3091502)Show SMILES CN1CCN(CC1)C(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cc(F)cc(N2CCCCC2=O)c1Cl |r| Show InChI InChI=1S/C24H28Cl2FN5O5S2/c1-30-8-10-31(11-9-30)24(35)16(14-28-23(34)18-5-6-20(25)38-18)29-39(36,37)19-13-15(27)12-17(22(19)26)32-7-3-2-4-21(32)33/h5-6,12-13,16,29H,2-4,7-11,14H2,1H3,(H,28,34)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50443846
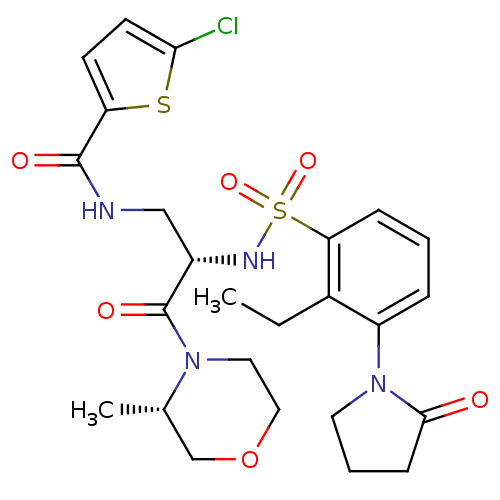
(CHEMBL3091527)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)N1CCOC[C@@H]1C)N1CCCC1=O |r| Show InChI InChI=1S/C25H31ClN4O6S2/c1-3-17-19(30-11-5-8-23(30)31)6-4-7-21(17)38(34,35)28-18(25(33)29-12-13-36-15-16(29)2)14-27-24(32)20-9-10-22(26)37-20/h4,6-7,9-10,16,18,28H,3,5,8,11-15H2,1-2H3,(H,27,32)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay |
J Med Chem 56: 9441-56 (2014)
Article DOI: 10.1021/jm4005835
BindingDB Entry DOI: 10.7270/Q20K2B0C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
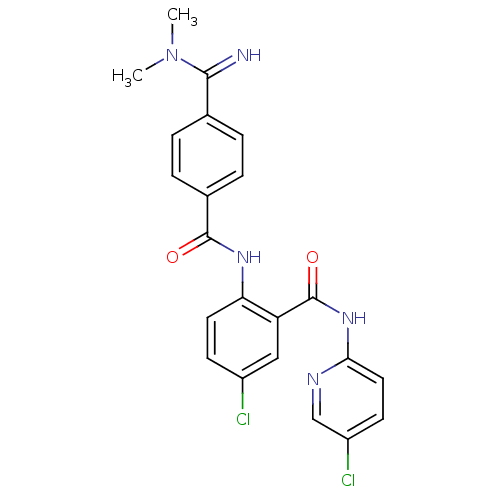
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data