

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112421 (US8623889, 420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50303233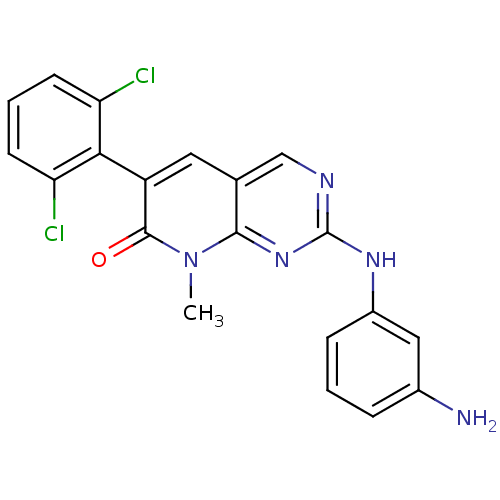 (2-(3-aminophenylamino)-6-(2,6-dichlorophenyl)-8-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Abl after 30 mins | Bioorg Med Chem 18: 6316-21 (2010) Article DOI: 10.1016/j.bmc.2010.07.021 BindingDB Entry DOI: 10.7270/Q2CZ384X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50378812 (CHEMBL1221411) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Abl after 30 mins | Bioorg Med Chem 18: 6316-21 (2010) Article DOI: 10.1016/j.bmc.2010.07.021 BindingDB Entry DOI: 10.7270/Q2CZ384X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112312 (US8623889, 311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112359 (US8623889, 358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112161 (US8623889, 159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112230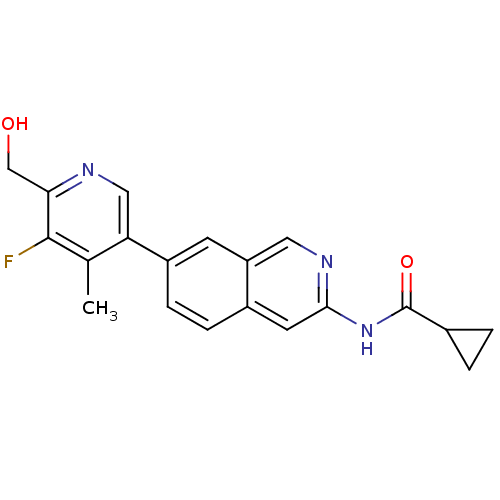 (US8623889, 228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112066 (US8623889, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112111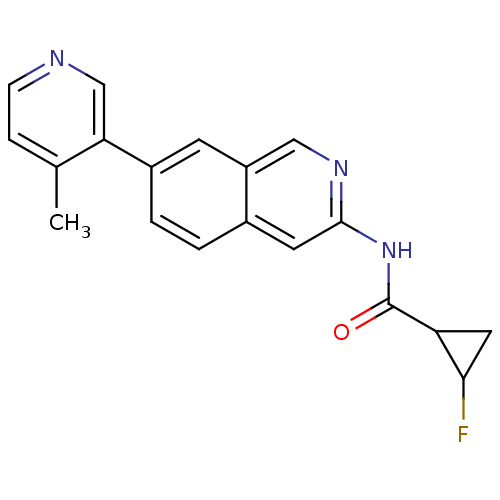 (US8623889, 109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112193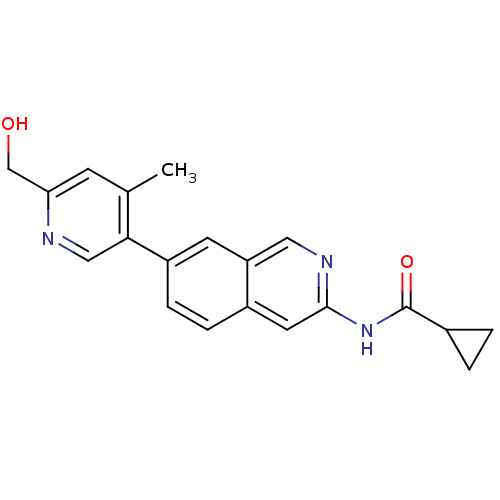 (US8623889, 191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112288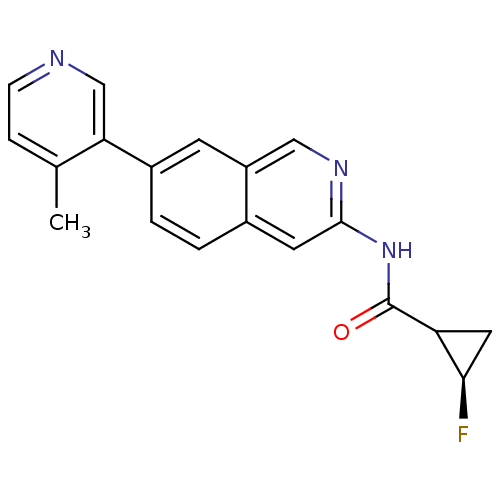 (US8623889, 286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112289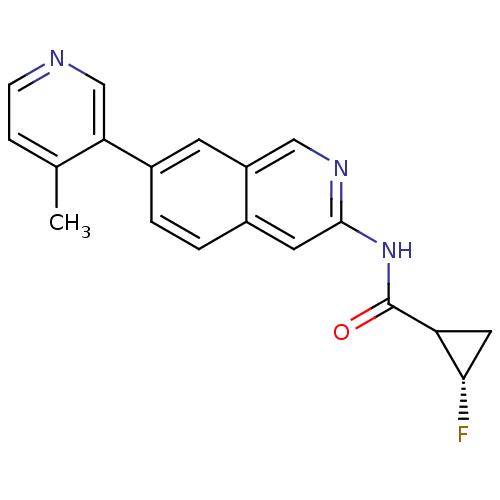 (US8623889, 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112251 (US8623889, 249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112274 (US8623889, 272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112106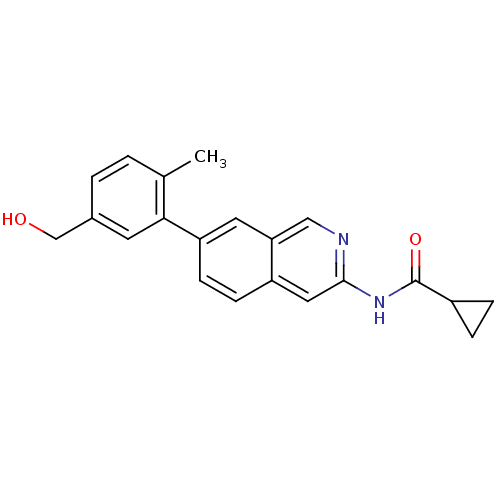 (US8623889, 104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112029 (US8623889, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112099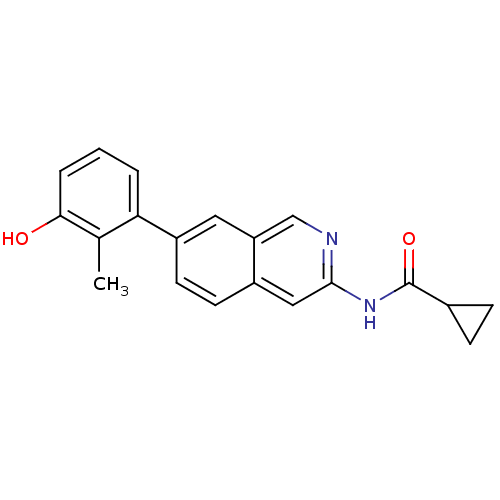 (US8623889, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112105 (US8623889, 103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112275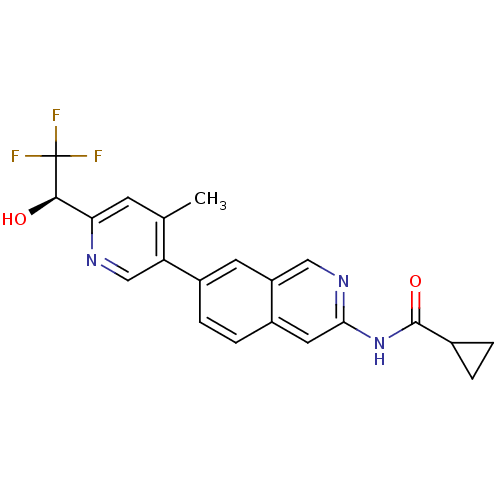 (US8623889, 273) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112192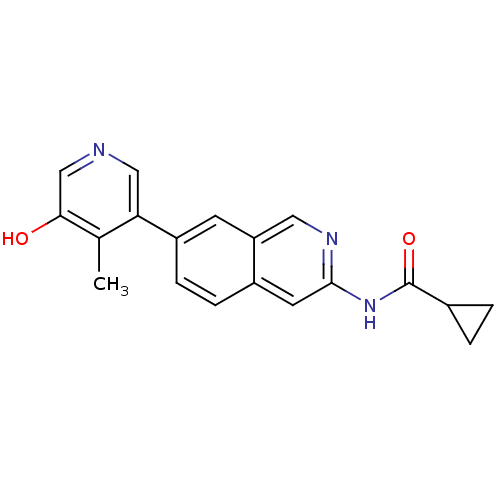 (US8623889, 190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112144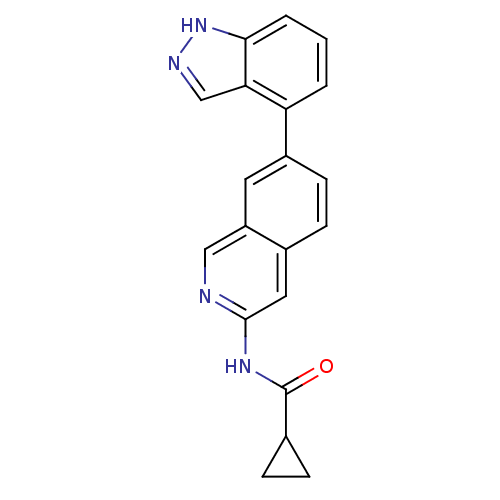 (US8623889, 142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112032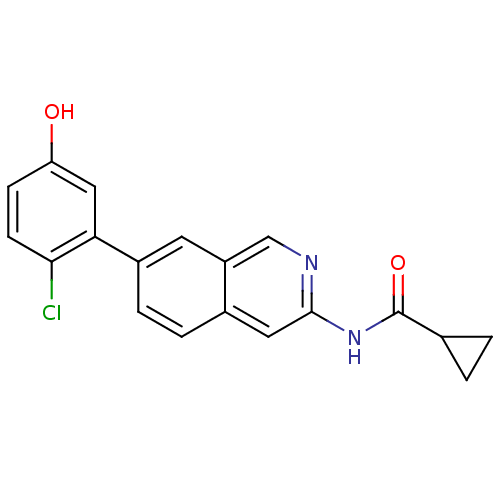 (US8623889, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112252 (US8623889, 250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112244 (US8623889, 242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112255 (US8623889, 253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112245 (US8623889, 243) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112254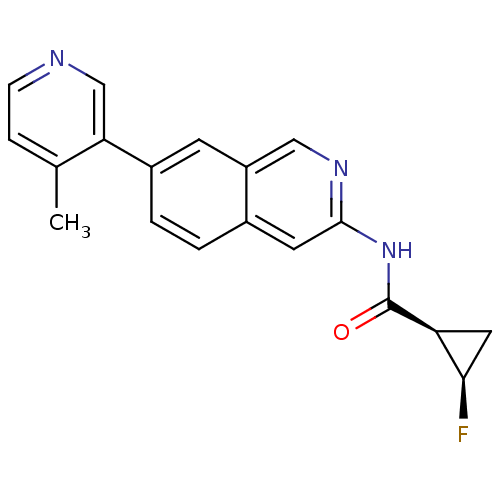 (US8623889, 252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112078 (US8623889, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50221547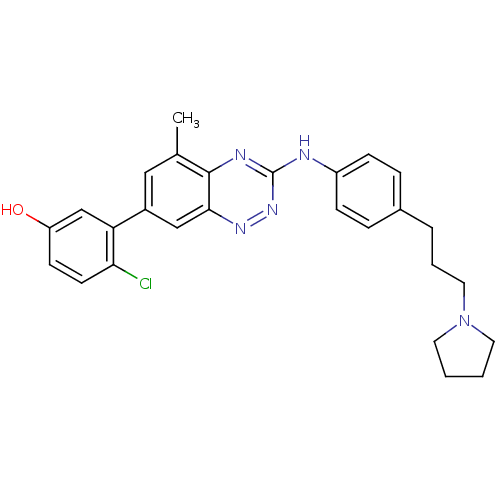 (4-chloro-3-(5-methyl-3-(4-(3-(pyrrolidin-1-yl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl | Bioorg Med Chem Lett 17: 5812-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.043 BindingDB Entry DOI: 10.7270/Q2M32VGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112289 (US8623889, 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112065 (US8623889, 63) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112194 (US8623889, 192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112157 (US8623889, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112288 (US8623889, 286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112379 (US8623889, 378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0709 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112418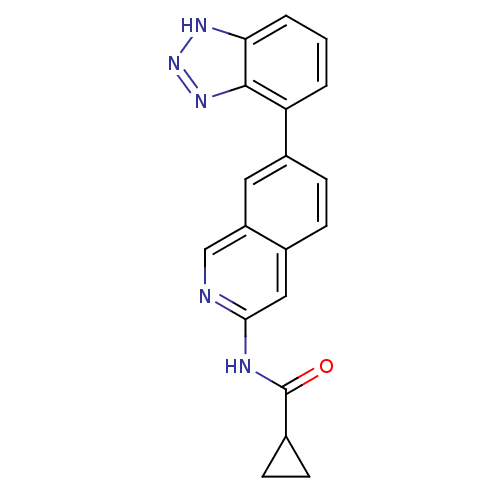 (US8623889, 417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0723 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112439 (US8623889, 438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0799 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112438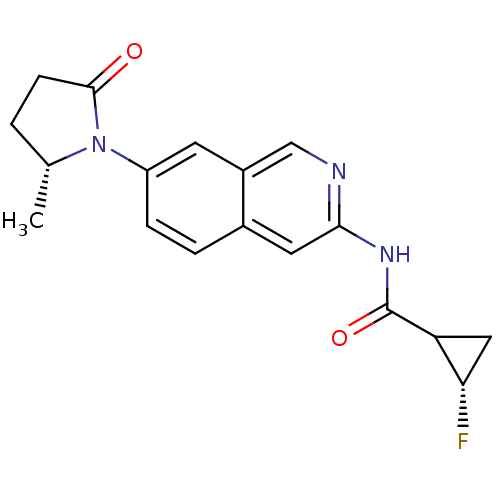 (US8623889, 437) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0799 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112330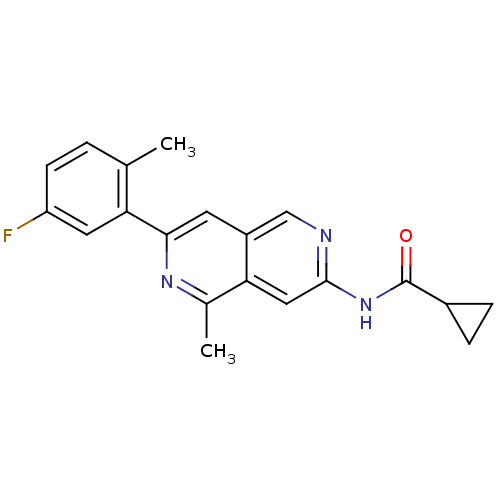 (US8623889, 329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112408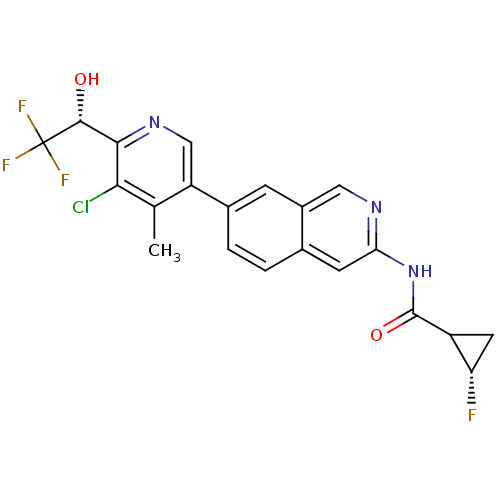 (US8623889, 407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0841 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112409 (US8623889, 408) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0841 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112425 (US8623889, 424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0896 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112191 (US8623889, 189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112224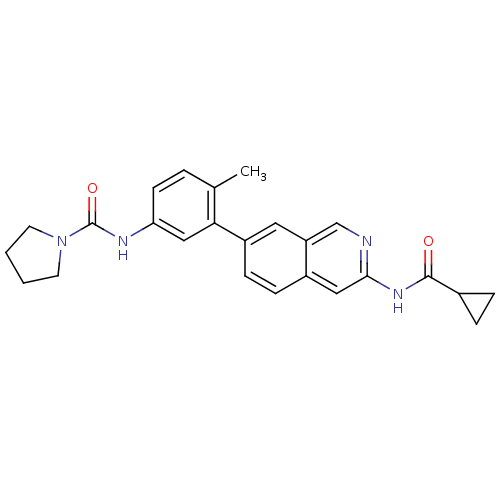 (US8623889, 222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112244 (US8623889, 242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112107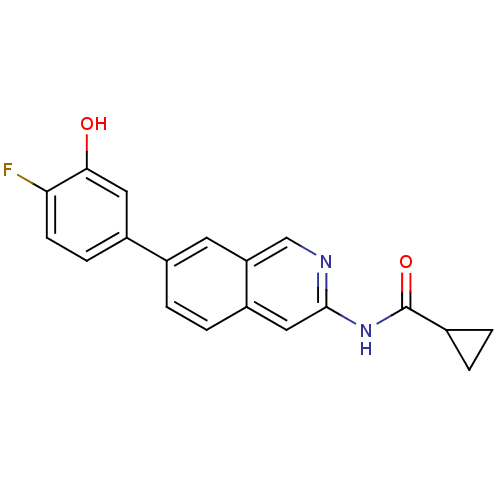 (US8623889, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112057 (US8623889, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112058 (US8623889, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112245 (US8623889, 243) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM112285 (US8623889, 283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... | US Patent US8623889 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 832 total ) | Next | Last >> |