

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250432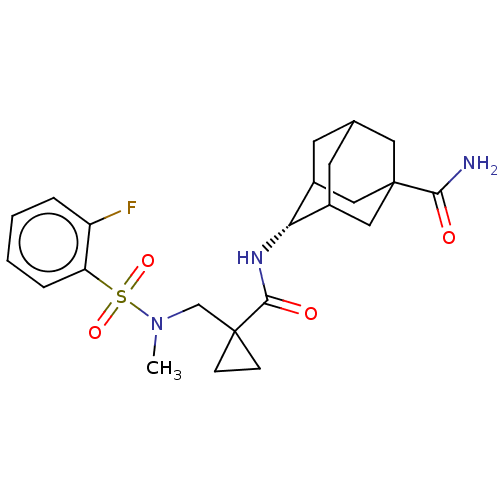 (US9464044, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317215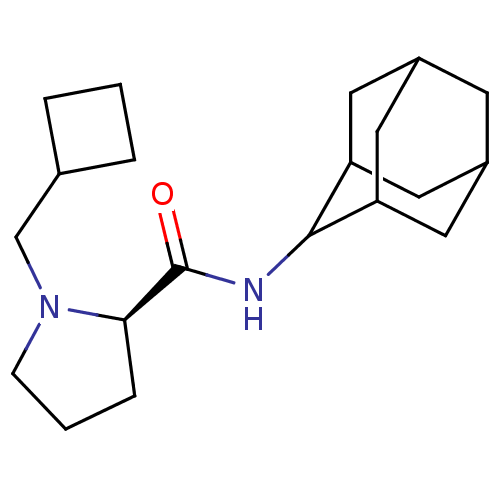 ((2R)-N-(adamantan-2-yl)-1-(cyclobutylmethyl)pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317220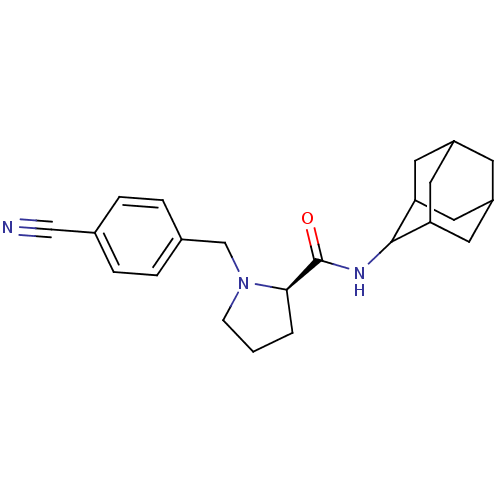 ((2R)-N-(adamantan-2-yl)-1-[(4-cyanophenyl)methyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317214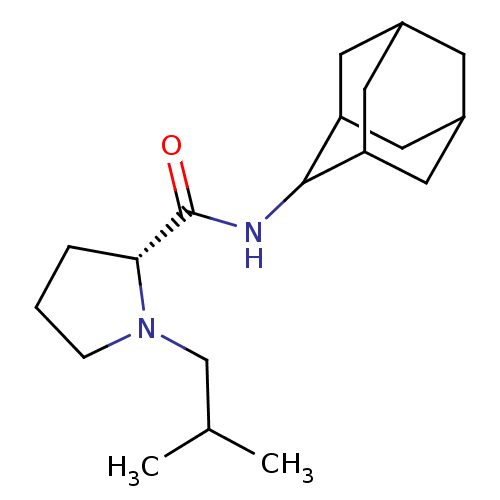 ((2R)-N-(adamantan-2-yl)-1-(2-methylpropyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317213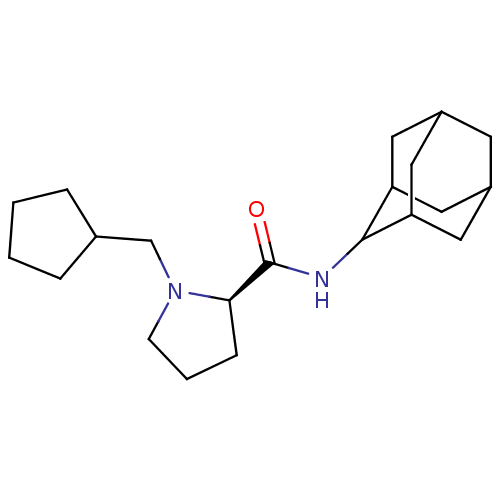 ((2R)-N-(adamantan-2-yl)-1-(cyclopentylmethyl)pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317226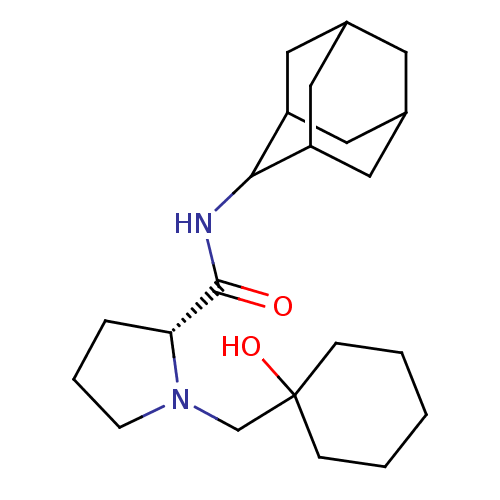 ((2R)-N-(adamantan-2-yl)-1-[(1-hydroxycyclohexyl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50392924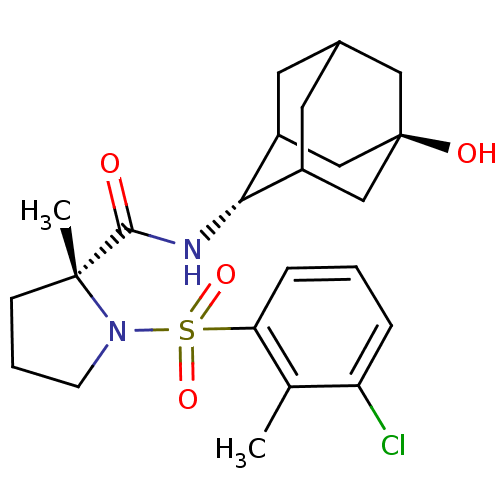 (CHEMBL2152223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 by scintillation proximity assay | ACS Med Chem Lett 3: 793-798 (2012) Article DOI: 10.1021/ml300144n BindingDB Entry DOI: 10.7270/Q2K938NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM167443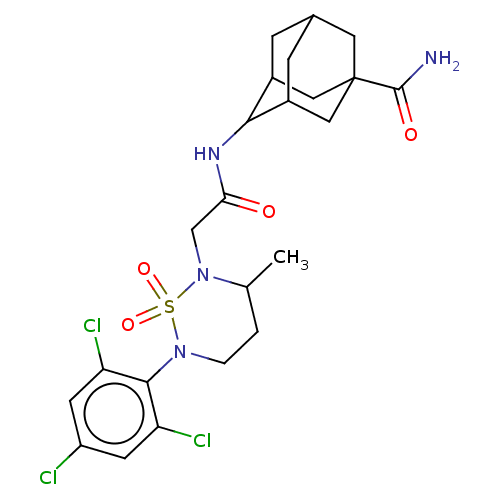 (US9073906, 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... | US Patent US9073906 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112149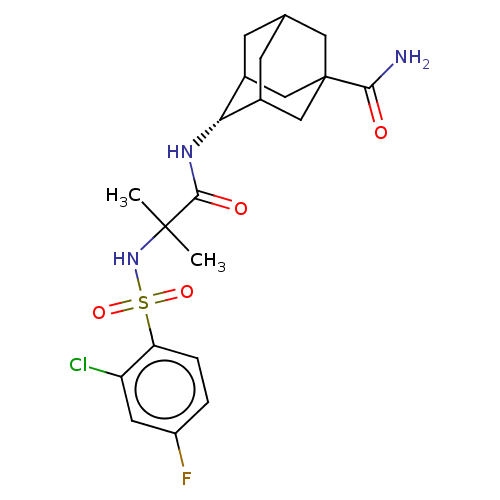 (CHEMBL3609877) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 25: 3501-6 (2015) Article DOI: 10.1016/j.bmcl.2015.06.099 BindingDB Entry DOI: 10.7270/Q2BP04KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50313285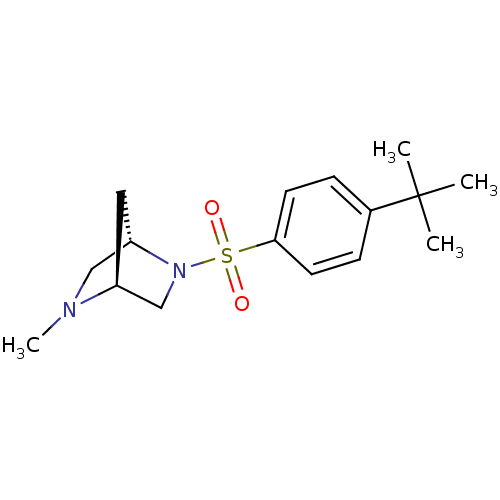 ((1S,4S)-2-(4-tert-butylphenylsulfonyl)-5-methyl-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cortisone from human 11beta-HSD1 expressed in baculovirus-infected Sf9 cells after 1 hr by scintillation proximity assay | Bioorg Med Chem Lett 20: 1551-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.082 BindingDB Entry DOI: 10.7270/Q2NV9JC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250460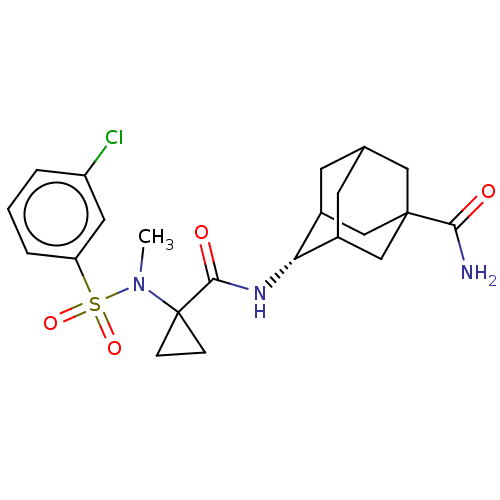 (US9464044, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM28365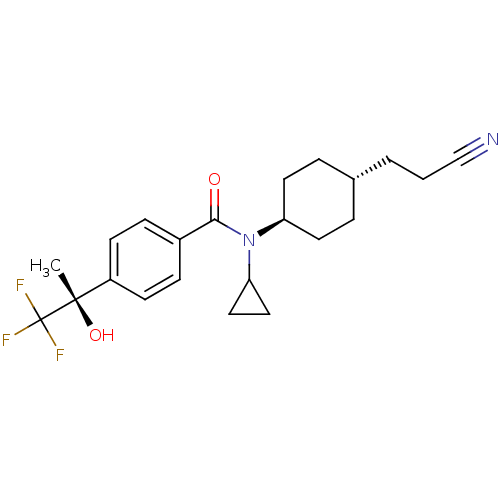 (CHEMBL512355 | N-[4-(2-cyanoethyl)cyclohexyl]-N-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human adipocytes | J Med Chem 51: 3953-60 (2008) Article DOI: 10.1021/jm800310g BindingDB Entry DOI: 10.7270/Q2P84CSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM167438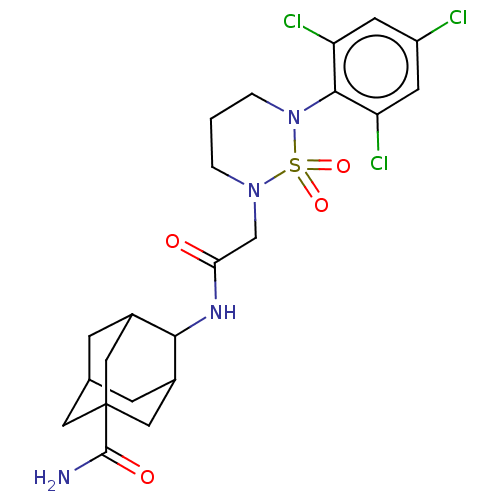 (US9073906, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... | US Patent US9073906 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112154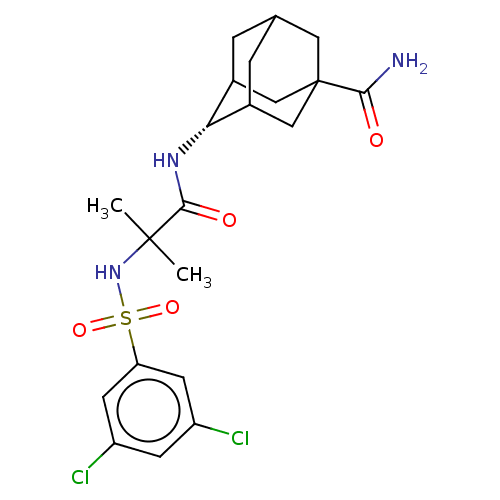 (CHEMBL3608403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 25: 3501-6 (2015) Article DOI: 10.1016/j.bmcl.2015.06.099 BindingDB Entry DOI: 10.7270/Q2BP04KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50313286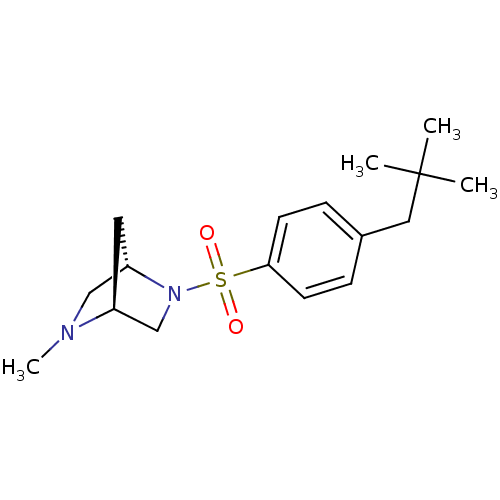 ((1S,4S)-2-methyl-5-(4-neopentylphenylsulfonyl)-2,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cortisone from mouse 11beta-HSD1 expressed in baculovirus-infected Sf9 cells after 1 hr by scintillation proximity assay | Bioorg Med Chem Lett 20: 1551-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.082 BindingDB Entry DOI: 10.7270/Q2NV9JC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50260704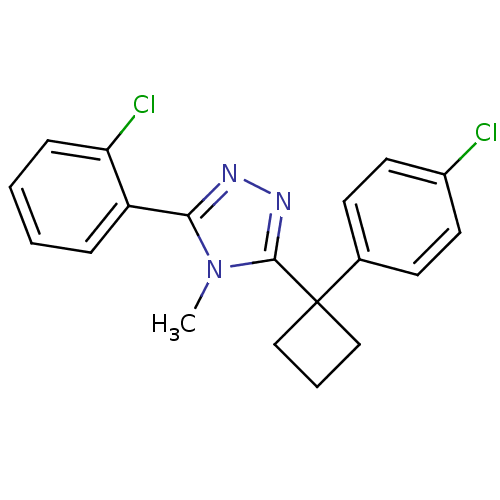 (3-(2-chlorophenyl)-5-(1-(4-chlorophenyl)cyclobutyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human 11beta HSD1 by SPA assay | Bioorg Med Chem Lett 18: 3405-11 (2008) Article DOI: 10.1016/j.bmcl.2008.04.013 BindingDB Entry DOI: 10.7270/Q2XP74QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239610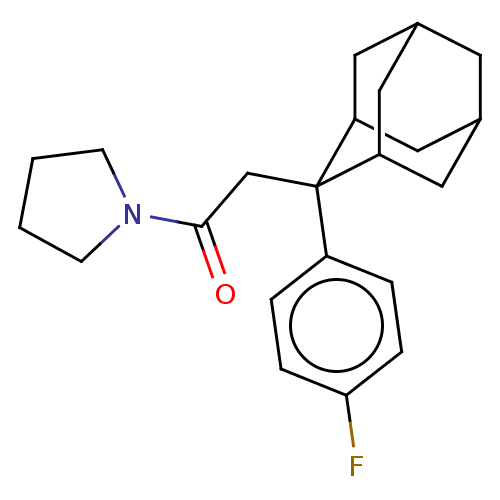 (CHEMBL4073961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM142001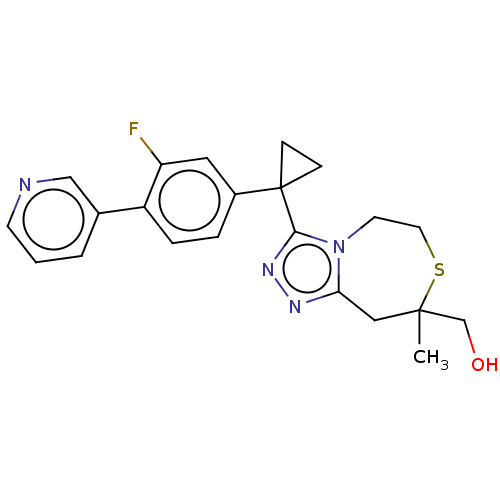 (US8927536, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A reaction was performed on a 384-well plate (Greiner Bio One) using a reaction volume of 24 ÁL, and all the samples were diluted with an assay buffe... | US Patent US8927536 (2015) BindingDB Entry DOI: 10.7270/Q2Q23XZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112151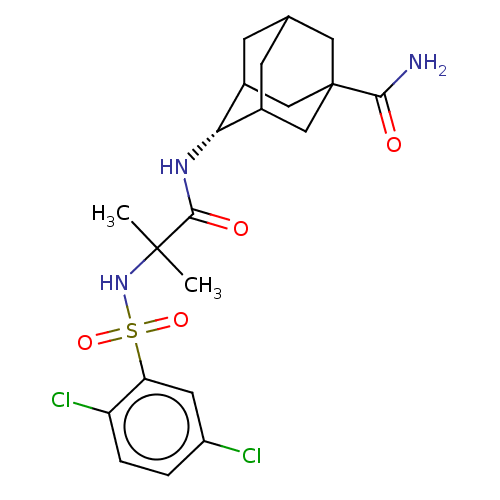 (CHEMBL3608400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 25: 3501-6 (2015) Article DOI: 10.1016/j.bmcl.2015.06.099 BindingDB Entry DOI: 10.7270/Q2BP04KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250418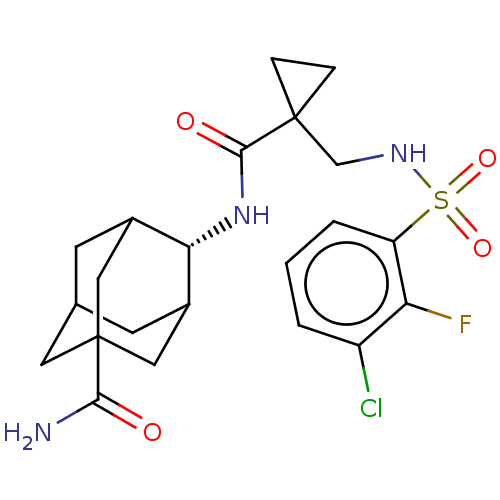 (US9464044, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107578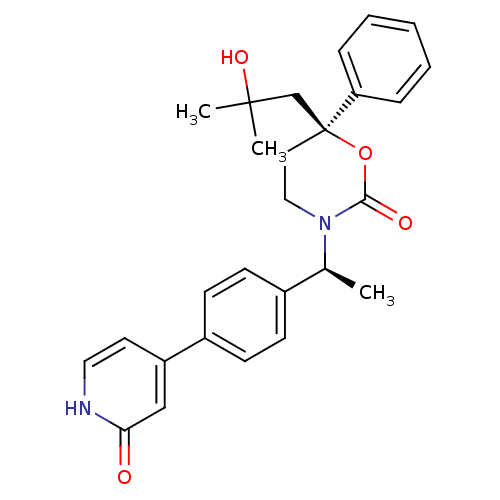 (US8575157, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317216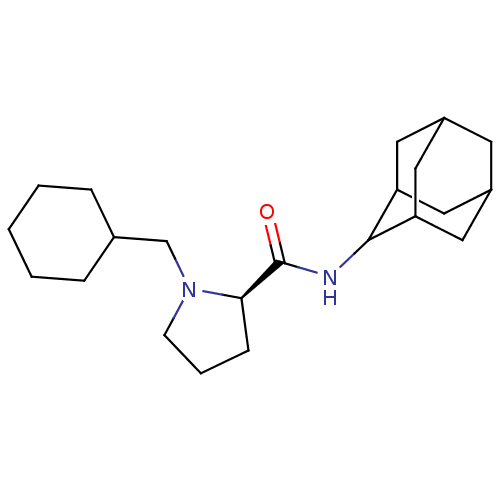 ((2R)-N-(adamantan-2-yl)-1-(cyclohexylmethyl)pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317217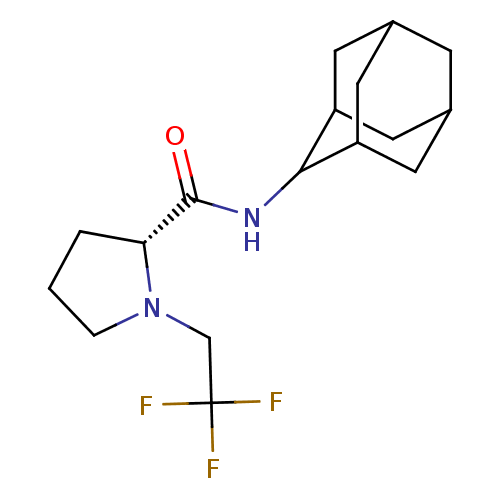 ((2R)-N-(adamantan-2-yl)-1-(2,2,2-trifluoroethyl)py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317221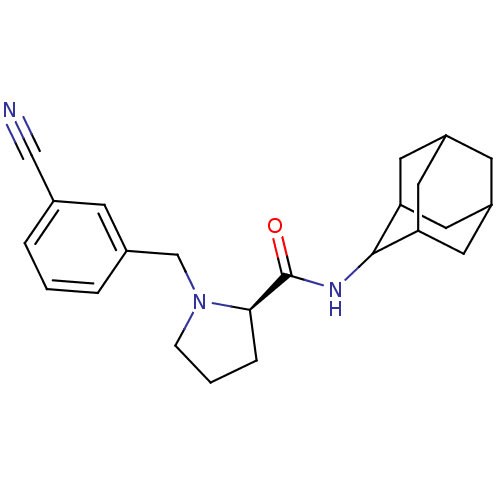 ((2R)-N-(adamantan-2-yl)-1-[(3-cyanophenyl)methyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317219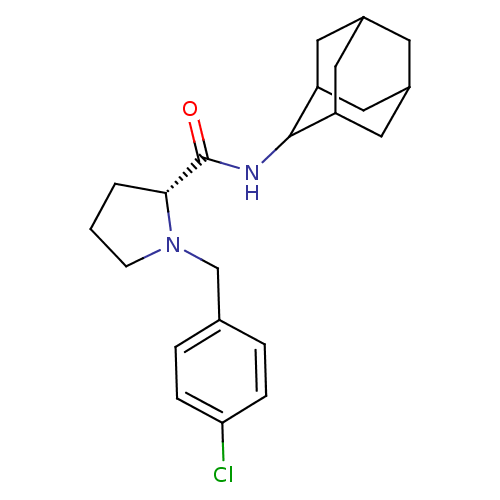 ((2R)-N-(adamantan-2-yl)-1-[(4-chlorophenyl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317218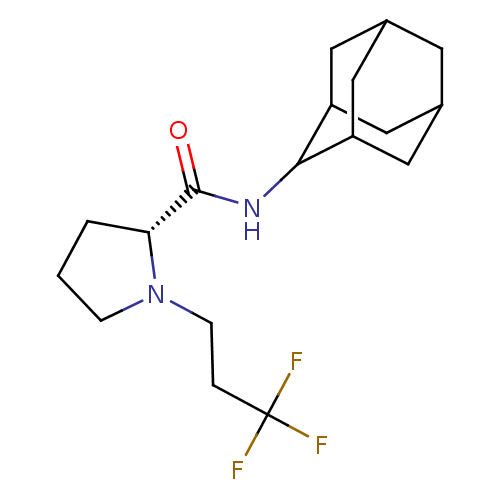 ((2R)-N-(adamantan-2-yl)-1-(3,3,3-trifluoropropyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50313286 ((1S,4S)-2-methyl-5-(4-neopentylphenylsulfonyl)-2,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cortisone from human 11beta-HSD1 expressed in baculovirus-infected Sf9 cells after 1 hr by scintillation proximity assay | Bioorg Med Chem Lett 20: 1551-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.082 BindingDB Entry DOI: 10.7270/Q2NV9JC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50260624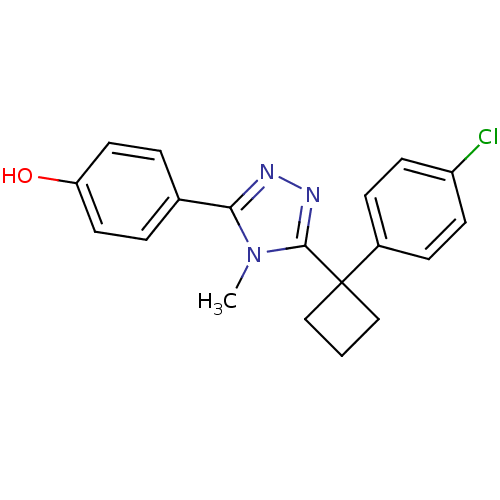 (4-(5-(1-(4-chlorophenyl)cyclobutyl)-4-methyl-4H-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human 11beta HSD1 by SPA assay | Bioorg Med Chem Lett 18: 3405-11 (2008) Article DOI: 10.1016/j.bmcl.2008.04.013 BindingDB Entry DOI: 10.7270/Q2XP74QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50317225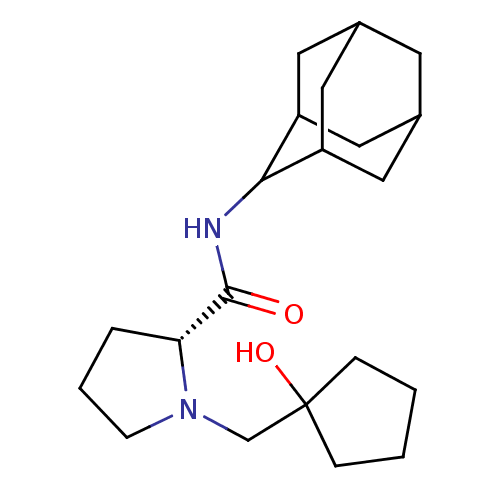 ((2R)-N-(adamantan-2-yl)-1-[(1-hydroxycyclopentyl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 2897-902 (2010) Article DOI: 10.1016/j.bmcl.2010.03.032 BindingDB Entry DOI: 10.7270/Q2NV9JDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112152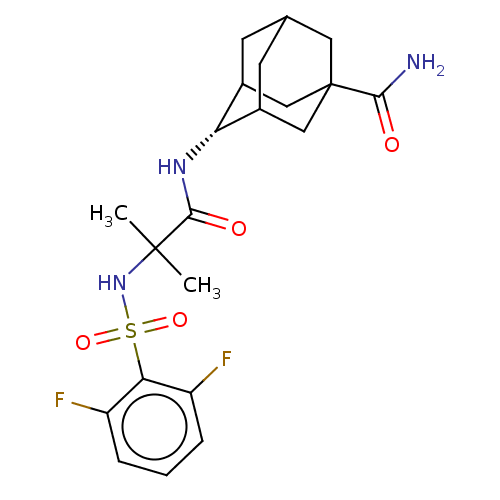 (CHEMBL3608401 | US9464044, 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50313285 ((1S,4S)-2-(4-tert-butylphenylsulfonyl)-5-methyl-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cortisone from mouse 11beta-HSD1 expressed in baculovirus-infected Sf9 cells after 1 hr by scintillation proximity assay | Bioorg Med Chem Lett 20: 1551-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.082 BindingDB Entry DOI: 10.7270/Q2NV9JC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50329315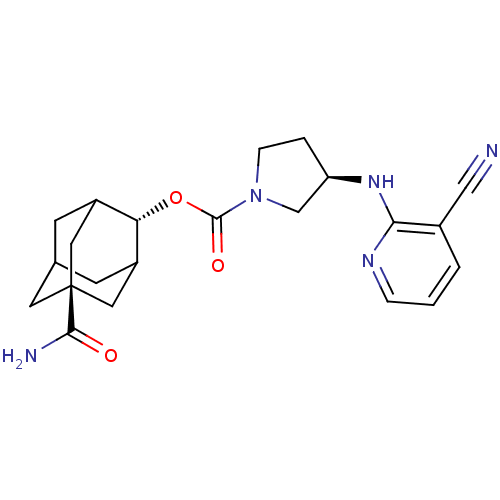 ((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader | Bioorg Med Chem Lett 20: 6725-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.142 BindingDB Entry DOI: 10.7270/Q2X92BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448693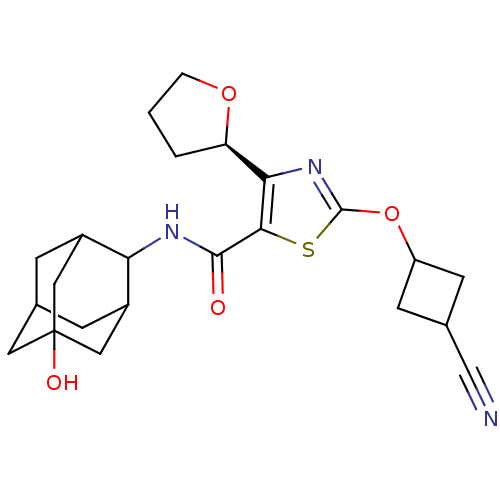 (CHEMBL3127857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448706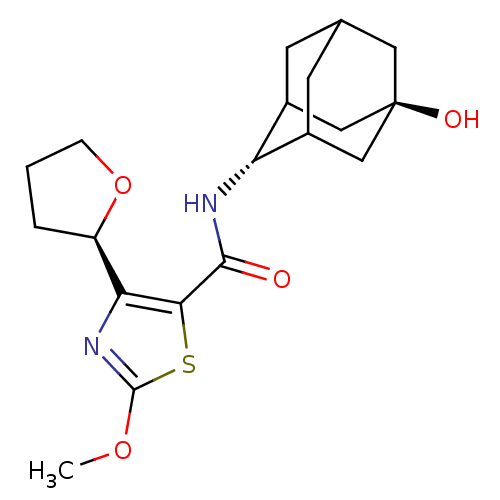 (CHEMBL3127854) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50392919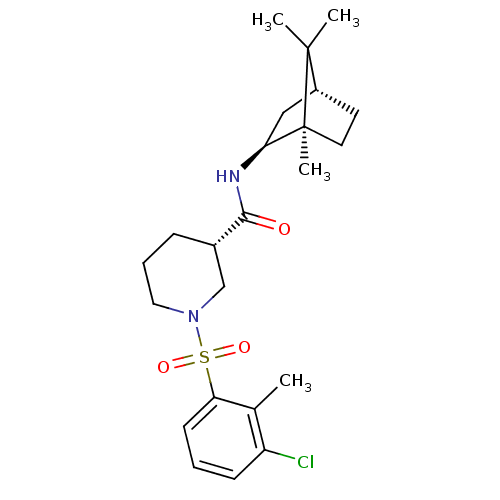 (CHEMBL2152217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 by scintillation proximity assay | ACS Med Chem Lett 3: 793-798 (2012) Article DOI: 10.1021/ml300144n BindingDB Entry DOI: 10.7270/Q2K938NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253406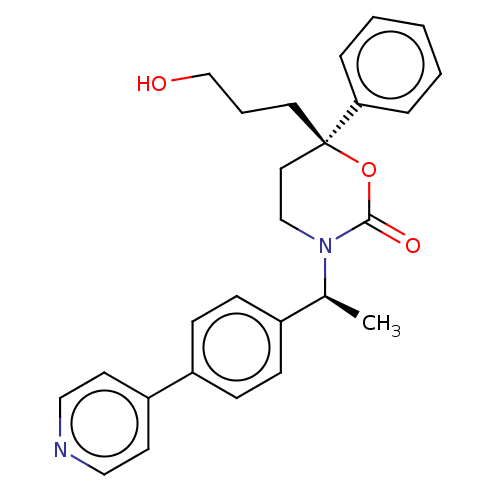 (CHEMBL4101787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250453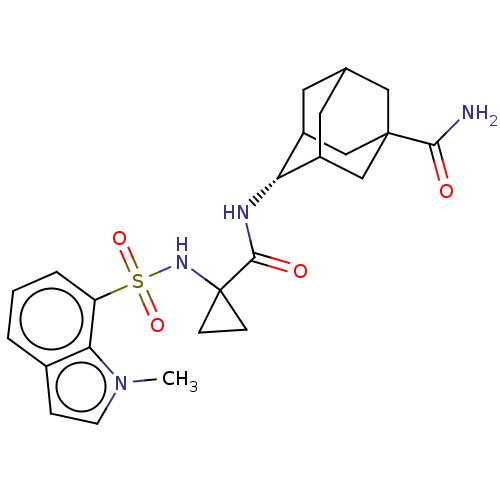 (US9464044, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353392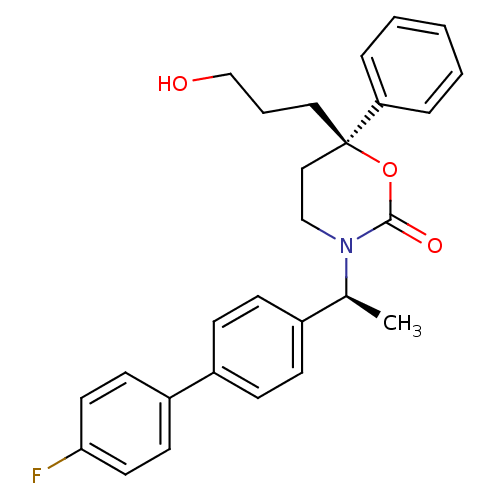 (CHEMBL1829761 | US8575157, 197 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of 11 beta-HSD1 in differentiated human adipocytes assessed as conversion of [3H]-cortisone to [3H]-cortisol after 10 mins by HPLC | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112152 (CHEMBL3608401 | US9464044, 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 25: 3501-6 (2015) Article DOI: 10.1016/j.bmcl.2015.06.099 BindingDB Entry DOI: 10.7270/Q2BP04KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50248471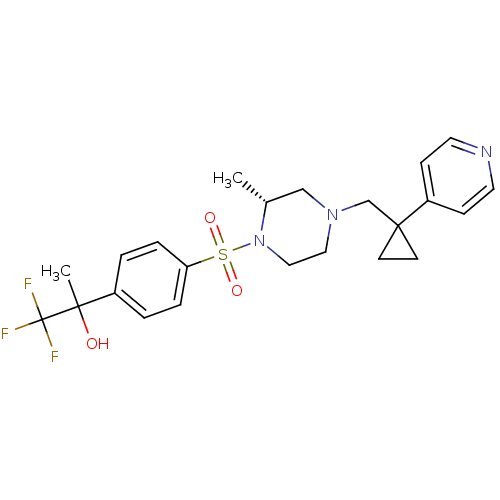 (1,1,1-trifluoro-2-(4-((R)-2-methyl-4-((1-(pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full length human recombinant 11beta-HSD1 expressed in baculovirus insect cell system assessed as conversion of [3H]cortisone to [3H]co... | Bioorg Med Chem Lett 19: 1522-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.114 BindingDB Entry DOI: 10.7270/Q2QC03BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM167440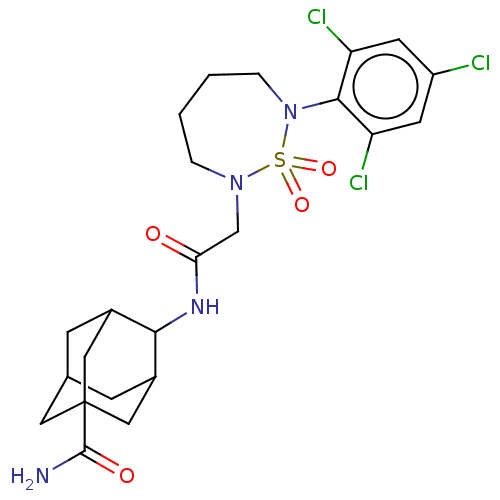 (US9073906, 122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description The in vitro inhibitory activities of the novel compounds to human 11β-HSD1 were evaluated in accordance with homogenous time-resolved fluoresce... | US Patent US9073906 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50017008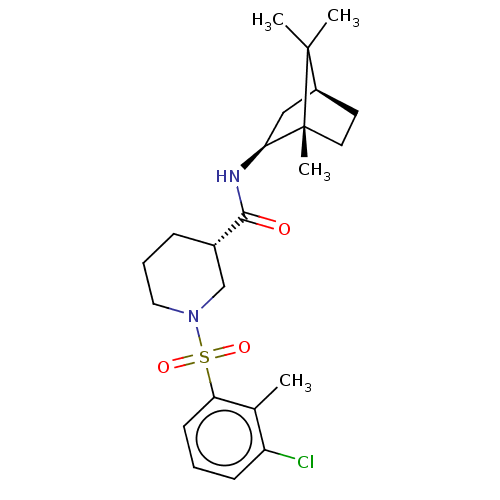 (CHEMBL3287025) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | J Med Chem 57: 4466-86 (2014) Article DOI: 10.1021/jm4014746 BindingDB Entry DOI: 10.7270/Q280546B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448731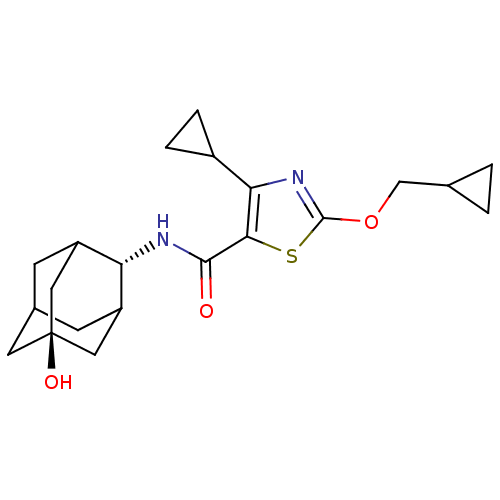 (CHEMBL3127868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448704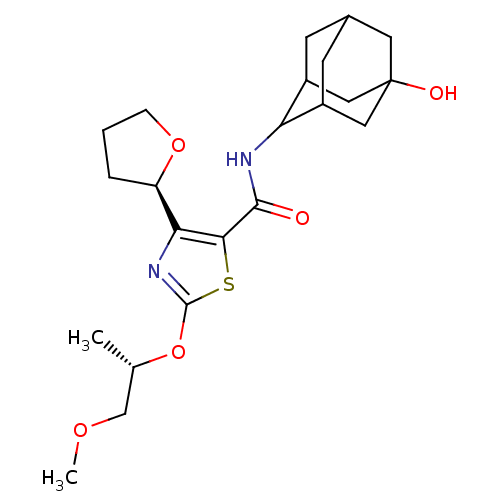 (CHEMBL3127856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM186151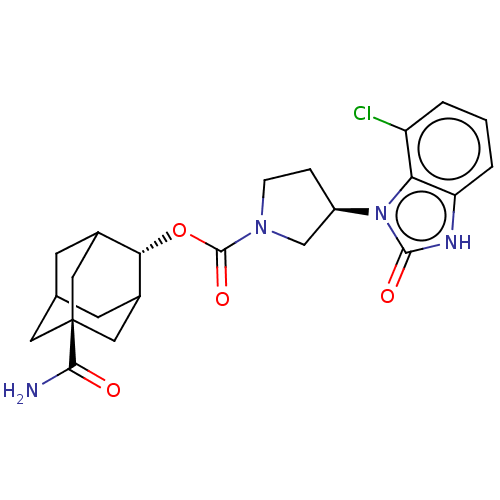 (US9163012, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Inhibition of microsomal preparations of 11.beta.-HSDl by compounds of the present invention, as described essentially previously (K. Solly, SS Mundt... | US Patent US9163012 (2015) BindingDB Entry DOI: 10.7270/Q2TD9W45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM186136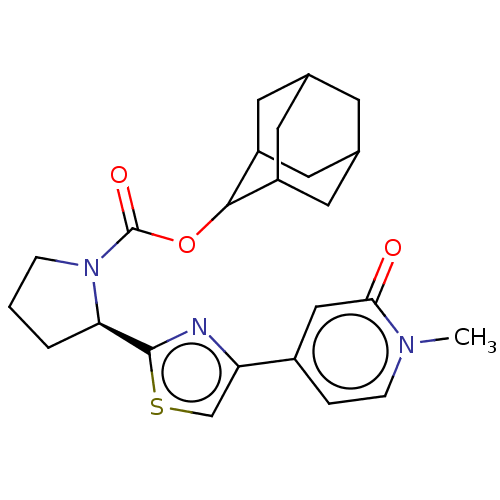 (US9163012, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Inhibition of microsomal preparations of 11.beta.-HSDl by compounds of the present invention, as described essentially previously (K. Solly, SS Mundt... | US Patent US9163012 (2015) BindingDB Entry DOI: 10.7270/Q2TD9W45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107475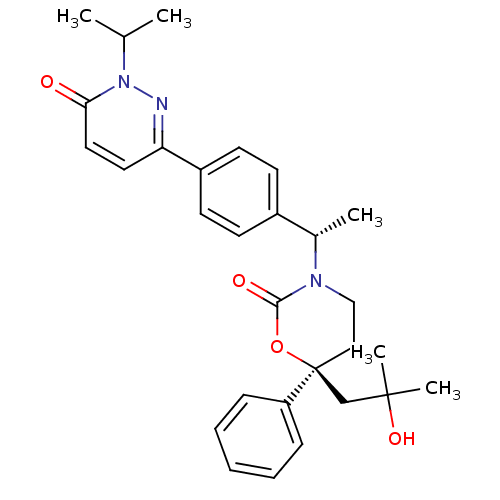 (US8592410, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448705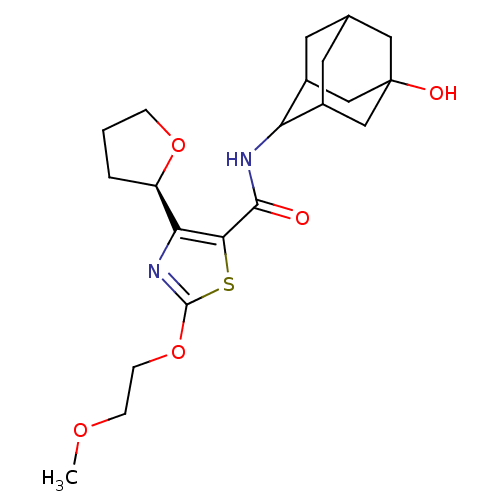 (CHEMBL3127855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50507376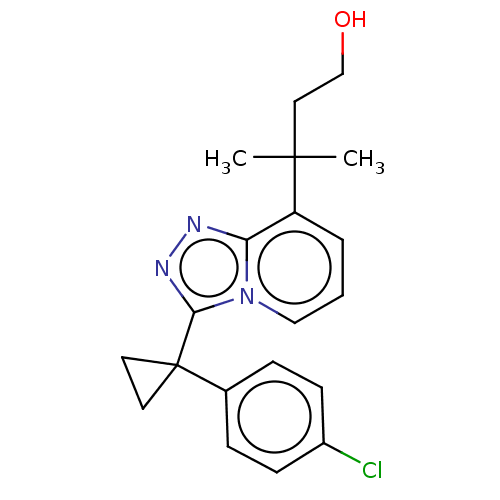 (CHEMBL4453347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... | ACS Med Chem Lett 9: 1170-1174 (2018) Article DOI: 10.1021/acsmedchemlett.8b00307 BindingDB Entry DOI: 10.7270/Q20R9SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50329313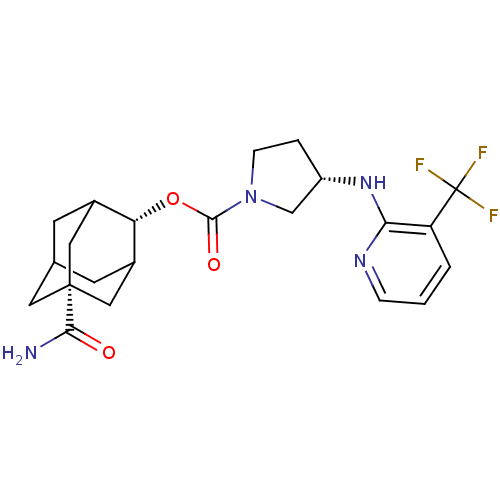 ((S)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader | Bioorg Med Chem Lett 20: 6725-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.142 BindingDB Entry DOI: 10.7270/Q2X92BJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5741 total ) | Next | Last >> |