

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50359991 (CHEMBL1927953) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assay | J Nat Prod 75: 599-604 (2012) Article DOI: 10.1021/np200831c BindingDB Entry DOI: 10.7270/Q27M090Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50359991 (CHEMBL1927953) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kunming Institute of Botany Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in human HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximity as... | J Nat Prod 74: 2571-5 (2011) Article DOI: 10.1021/np200755t BindingDB Entry DOI: 10.7270/Q2P26ZJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50185127 ((3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 | Eur J Med Chem 65: 403-14 (2013) Article DOI: 10.1016/j.ejmech.2013.05.010 BindingDB Entry DOI: 10.7270/Q2JS9RV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 overexpressed in microsomal fraction of HEK293 cells using [3H]-cortisol as substrate by scintillation proximity assa... | J Nat Prod 78: 330-4 (2015) Article DOI: 10.1021/np500896n BindingDB Entry DOI: 10.7270/Q2930VWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China. Curated by ChEMBL | Assay Description Inhibition of full-length human 11beta-HSD2 expressed in HEK293 microsomal fraction using [3H]cortisone as substrate after 2 hr by scintillation prox... | Eur J Med Chem 135: 324-338 (2017) Article DOI: 10.1016/j.ejmech.2017.04.059 BindingDB Entry DOI: 10.7270/Q2CF9SK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329192 ((3beta,18beta,20beta)-3-Acetoxy-N-methyl-N-hydroxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329192 ((3beta,18beta,20beta)-3-Acetoxy-N-methyl-N-hydroxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50174298 (3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 assessed as inhibition of conversion of radiolabeled cortisone to radiolabeled cortisol by cell-based assa... | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329187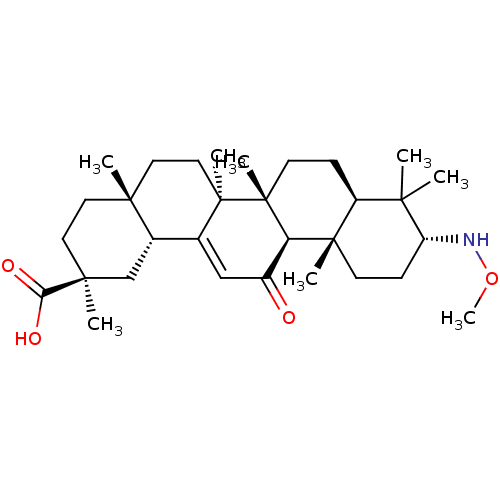 ((3alpha,18beta,20beta)-3-Methoxyamino-11-oxo-olean...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50340378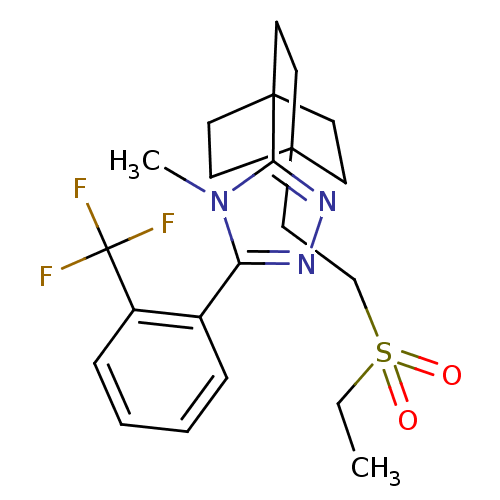 (3-(4-(2-(ethylsulfonyl)ethyl)bicyclo[2.2.2]octan-1...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 assessed as inhibition of conversion of radiolabeled cortisone to radiolabeled cortisol by cell-based assa... | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50340432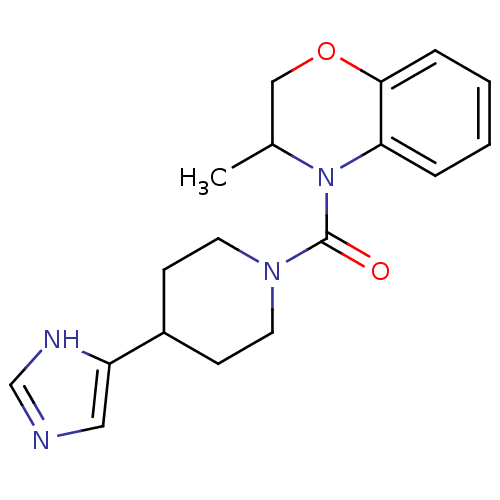 ((4-(1H-imidazol-4-yl)piperidin-1-yl)(3-methyl-2H-b...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D, Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 | Bioorg Med Chem Lett 21: 2244-51 (2011) Article DOI: 10.1016/j.bmcl.2011.02.111 BindingDB Entry DOI: 10.7270/Q26H4HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642875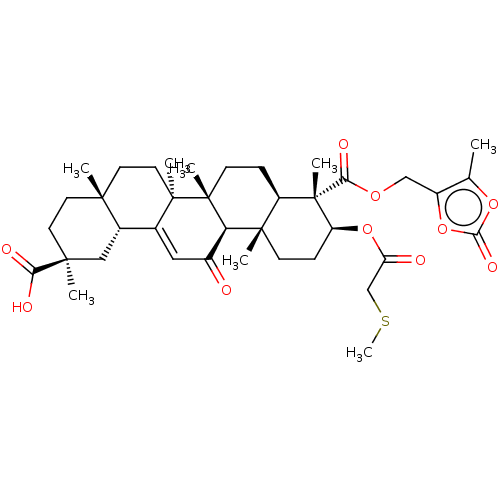 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-2,4a,6a...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50112133 (CHEMBL3609861 | US9464044, 71) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 | Bioorg Med Chem Lett 25: 3501-6 (2015) Article DOI: 10.1016/j.bmcl.2015.06.099 BindingDB Entry DOI: 10.7270/Q2BP04KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50340431 ((4-(1H-imidazol-4-yl)piperidin-1-yl)(3,4-dihydroqu...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-aventis R&D, Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 | Bioorg Med Chem Lett 21: 2244-51 (2011) Article DOI: 10.1016/j.bmcl.2011.02.111 BindingDB Entry DOI: 10.7270/Q26H4HQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50306423 (2-{1'-[(adamantan-2-yl)carbamoyl]-5-methyl-2,3-dih...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD2 | Bioorg Med Chem Lett 20: 881-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.082 BindingDB Entry DOI: 10.7270/Q2KK9BWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339805 ((3beta,18beta,20beta)-[3-(1,2,4-Oxadiazole-5(2H)-o...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339809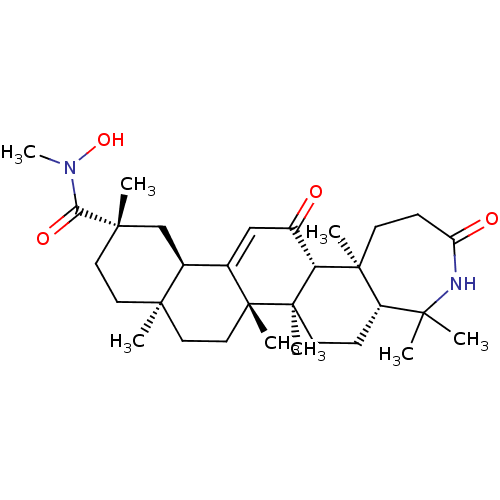 ((5aR,7aR,7bS,9aS,12S,13aR,15aR,15bS)-2,3,4,5,5a,6,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642880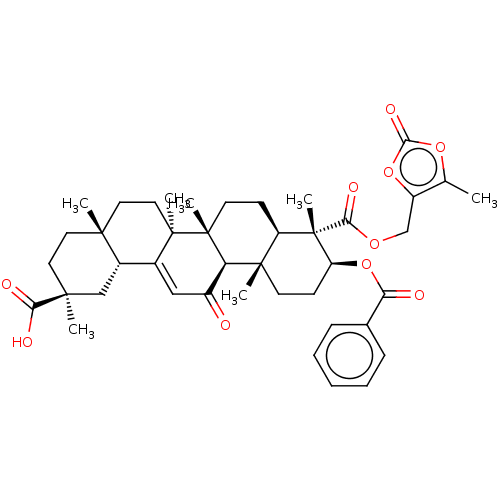 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-10-(Ben...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329188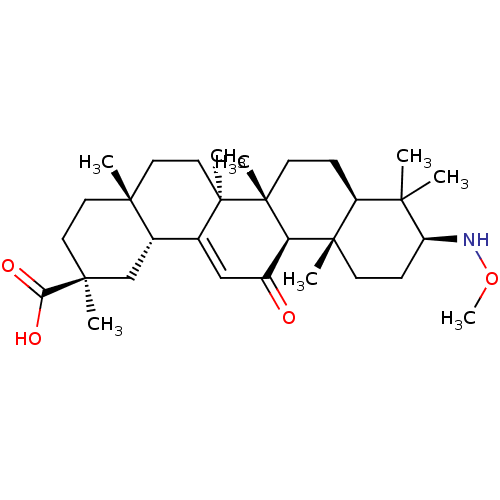 ((3beta,18beta,20beta)-3-Methoxyamino-11-oxo-olean-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339811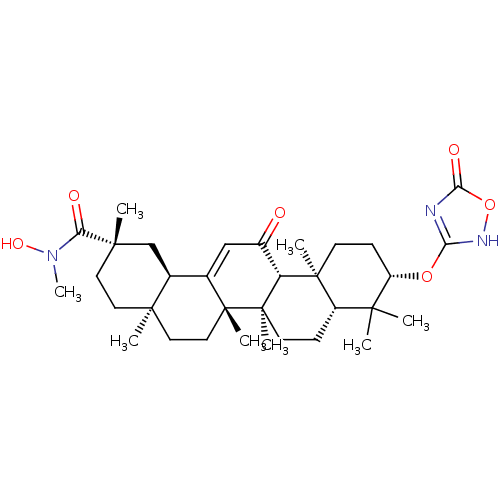 ((3beta,18beta,20beta)-N-Hydroxy-N-methyl-[3-(1,2,4...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339812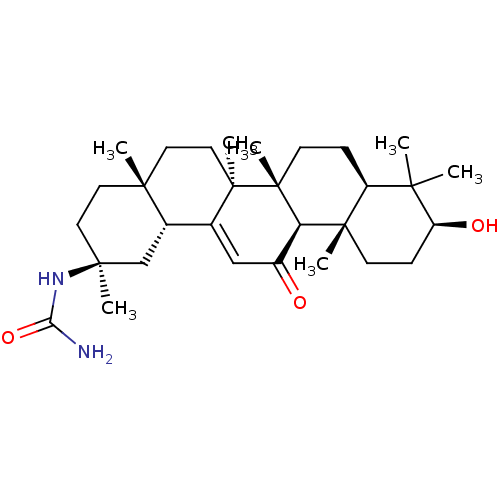 (CHEMBL1689283 | N-[(3beta,18beta,20beta)-3-Hydroxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50193790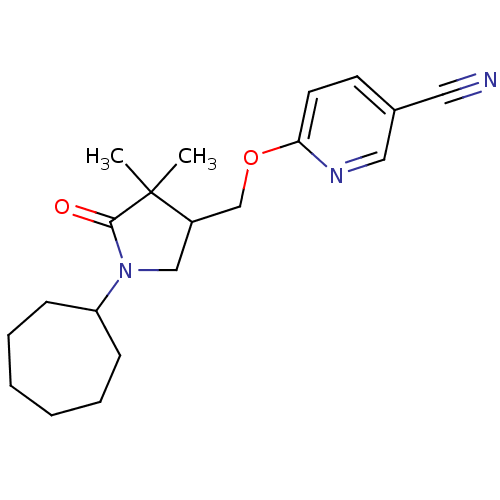 (6-((1-cycloheptyl-4,4-dimethyl-5-oxopyrrolidin-3-y...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 | Bioorg Med Chem Lett 16: 5555-60 (2006) Article DOI: 10.1016/j.bmcl.2006.08.034 BindingDB Entry DOI: 10.7270/Q25X28K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642882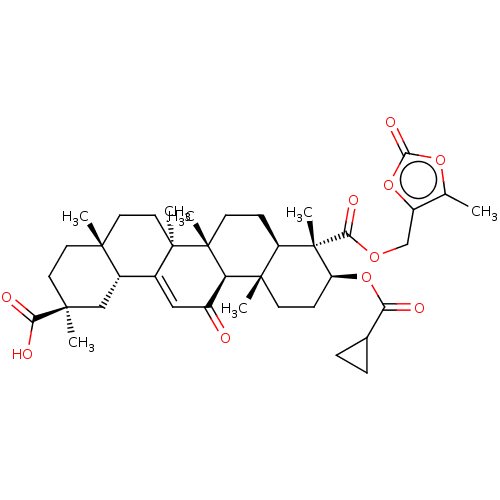 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-10-((Cy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50435691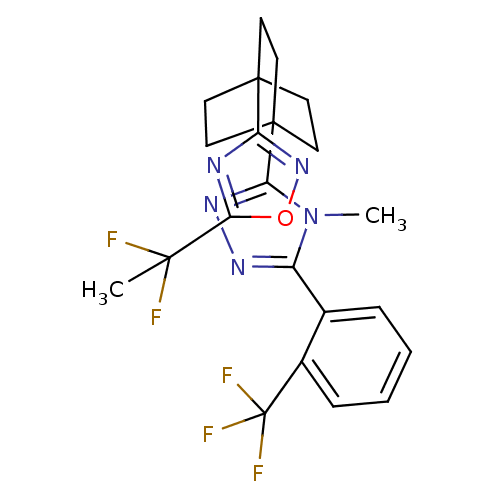 (CHEMBL2391968) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 assessed as inhibition of conversion of radiolabeled cortisone to radiolabeled cortisol by cell-based assa... | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50340386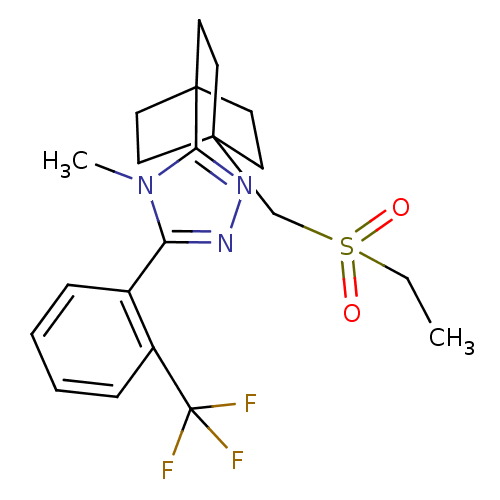 (3-(4-(ethylsulfonylmethyl)bicyclo[2.2.2]octan-1-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 assessed as inhibition of conversion of radiolabeled cortisone to radiolabeled cortisol by cell-based assa... | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642894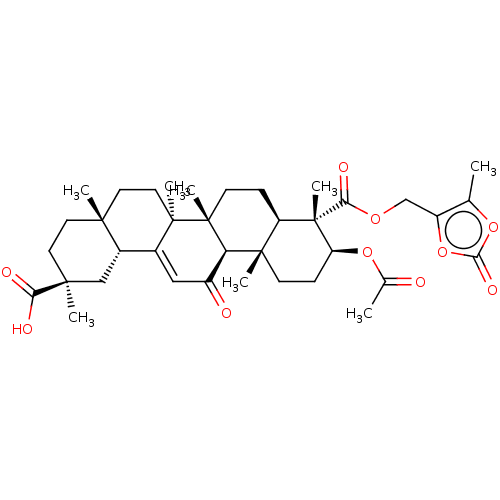 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-10-Acet...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642889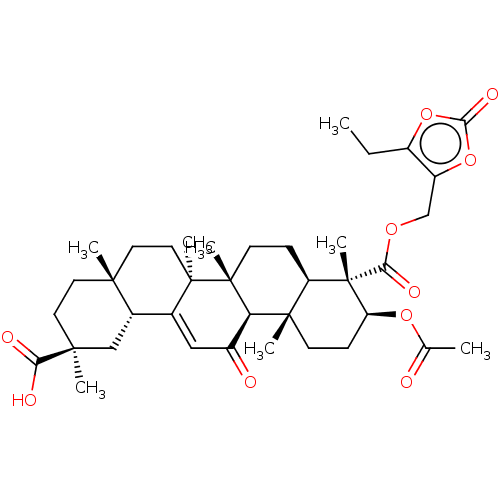 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-10-acet...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339808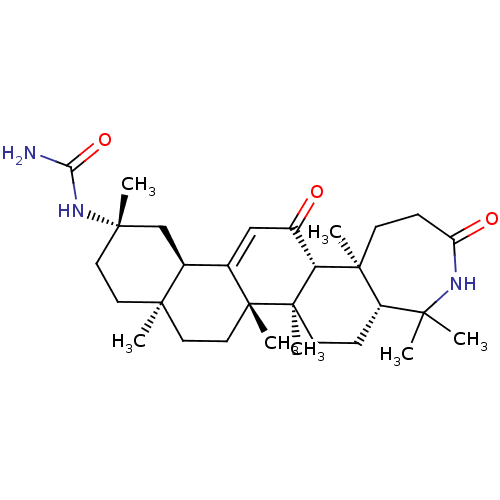 (CHEMBL1689279 | N-[(5aR,7aR,7bS,9aS,12S,13aR,15aR,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339813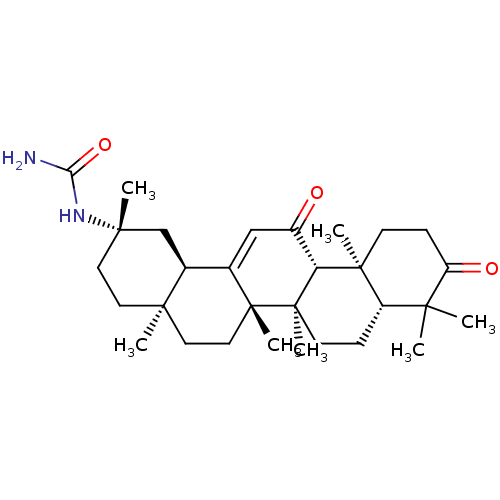 (CHEMBL1689284 | N-[(3beta,18beta,20beta)-3,11-Diox...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188387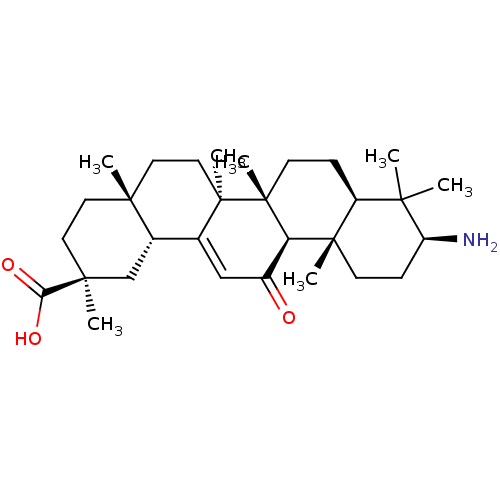 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-amino-2...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329193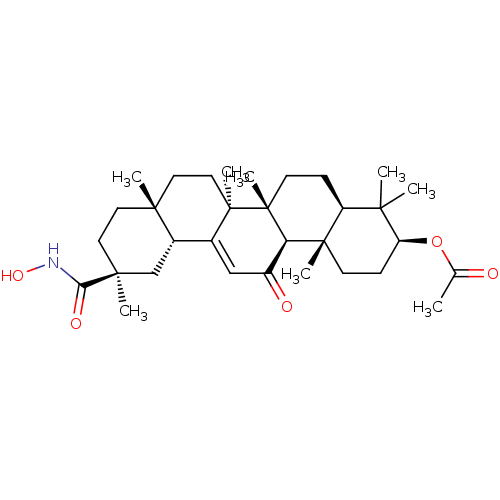 ((3beta,18beta,20beta)-3-Acetoxy-N-hydroxy-11-oxo-o...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642901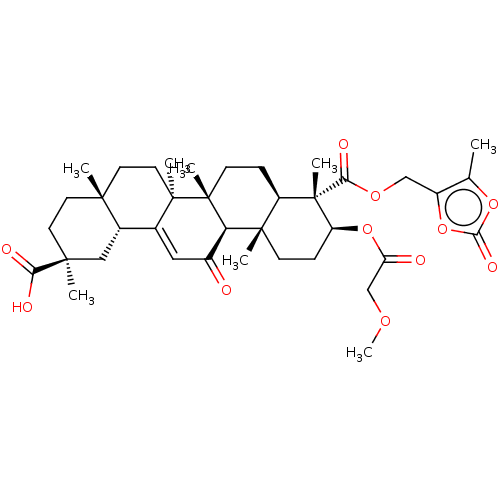 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-10-[(2-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642893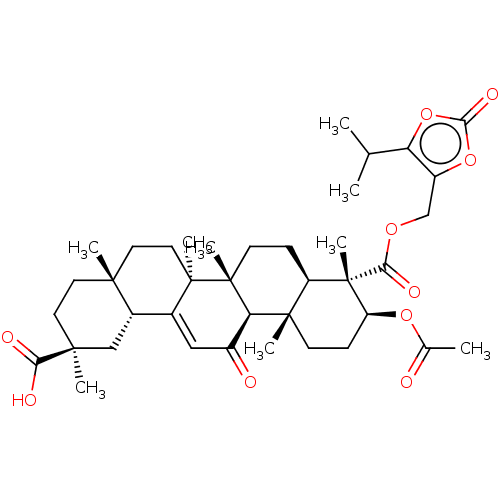 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-10-acet...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642892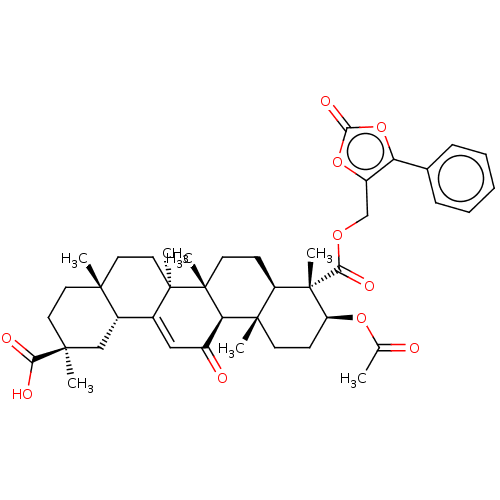 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-10-(ace...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Mus musculus (mouse)) | BDBM50247012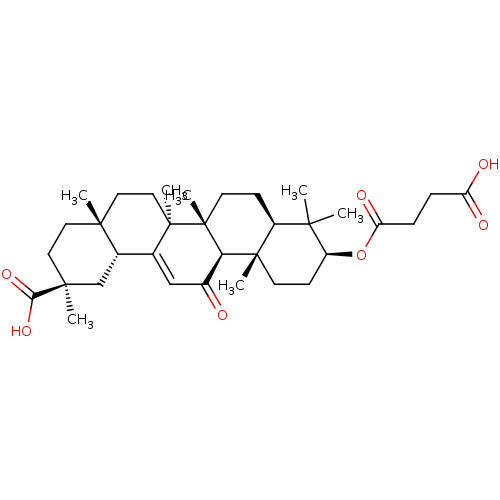 (3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50277179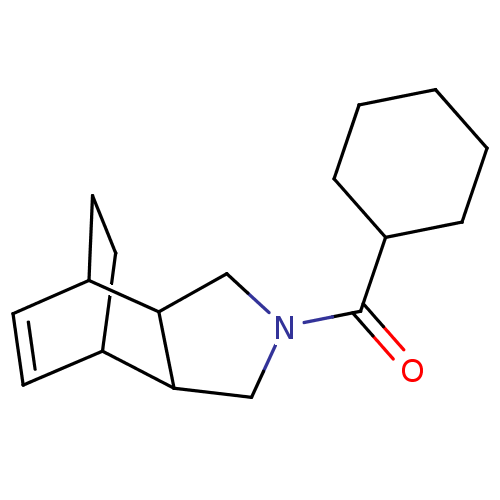 (CHEMBL4160328) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human full-length 11beta-HSD2 expressed in HEK293 cells using cortisol as substrate after 2 hrs by LC-MS analysis | Eur J Med Chem 139: 412-428 (2017) Article DOI: 10.1016/j.ejmech.2017.08.003 BindingDB Entry DOI: 10.7270/Q2JM2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50277198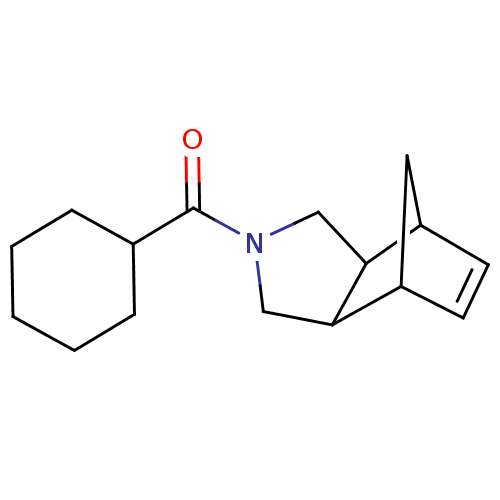 (CHEMBL4163698) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human full-length 11beta-HSD2 expressed in HEK293 cells using cortisol as substrate after 2 hrs by LC-MS analysis | Eur J Med Chem 139: 412-428 (2017) Article DOI: 10.1016/j.ejmech.2017.08.003 BindingDB Entry DOI: 10.7270/Q2JM2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50277202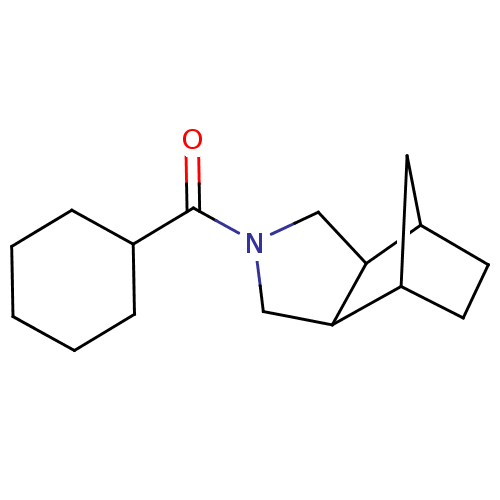 (CHEMBL4174368) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human full-length 11beta-HSD2 expressed in HEK293 cells using cortisol as substrate after 2 hrs by LC-MS analysis | Eur J Med Chem 139: 412-428 (2017) Article DOI: 10.1016/j.ejmech.2017.08.003 BindingDB Entry DOI: 10.7270/Q2JM2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50277203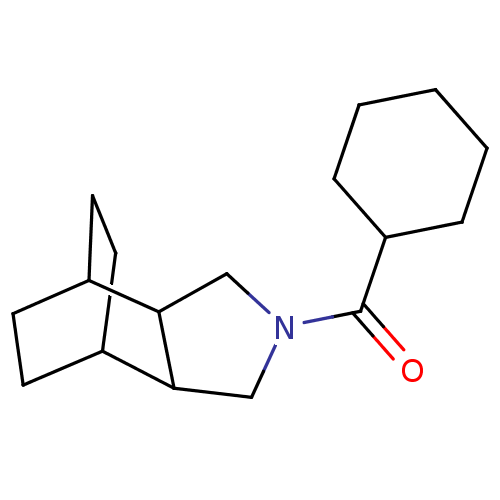 (CHEMBL4170932) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human full-length 11beta-HSD2 expressed in HEK293 cells using cortisol as substrate after 2 hrs by LC-MS analysis | Eur J Med Chem 139: 412-428 (2017) Article DOI: 10.1016/j.ejmech.2017.08.003 BindingDB Entry DOI: 10.7270/Q2JM2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50277178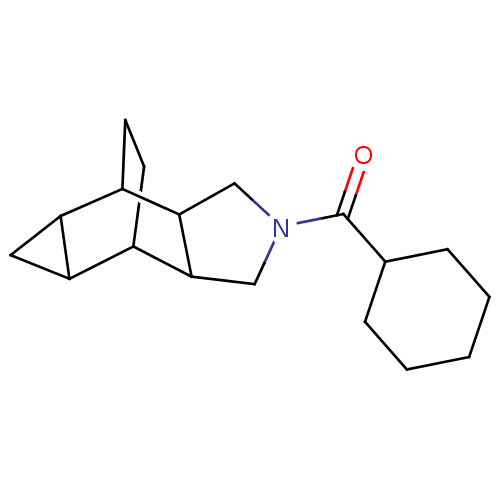 (CHEMBL4162610) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human full-length 11beta-HSD2 expressed in HEK293 cells using cortisol as substrate after 2 hrs by LC-MS analysis | Eur J Med Chem 139: 412-428 (2017) Article DOI: 10.1016/j.ejmech.2017.08.003 BindingDB Entry DOI: 10.7270/Q2JM2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642877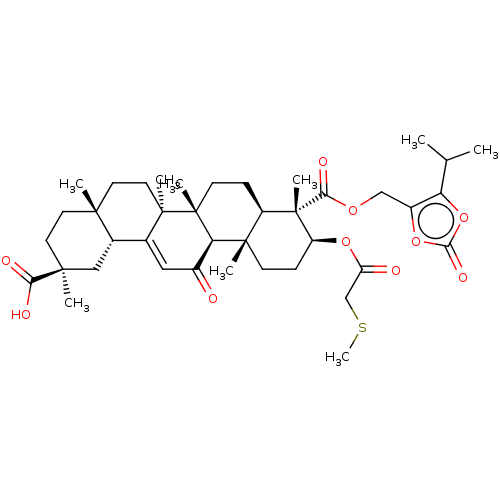 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-2,4a,6a...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339806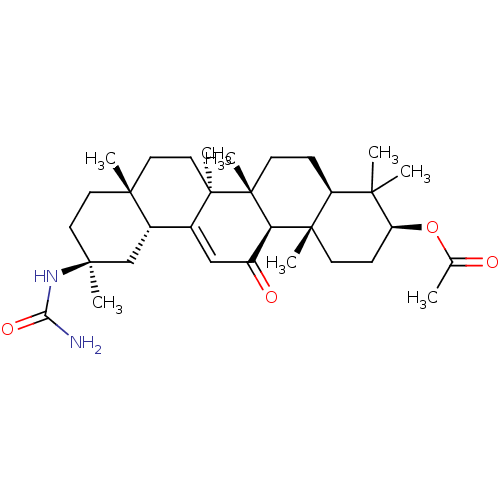 ((3beta,18beta,20beta)-N-[-3-(Acetoxy)-11-oxo-30-no...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329195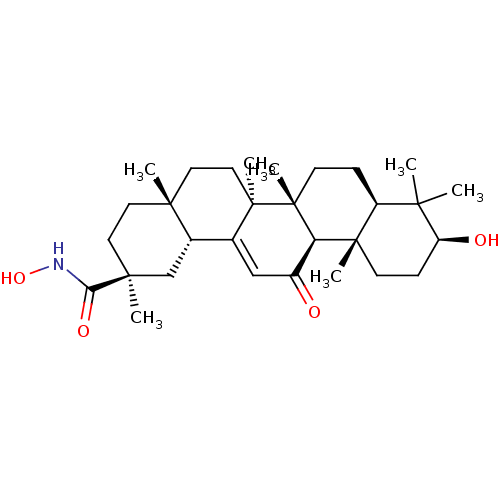 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-N,10-dihyd...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642909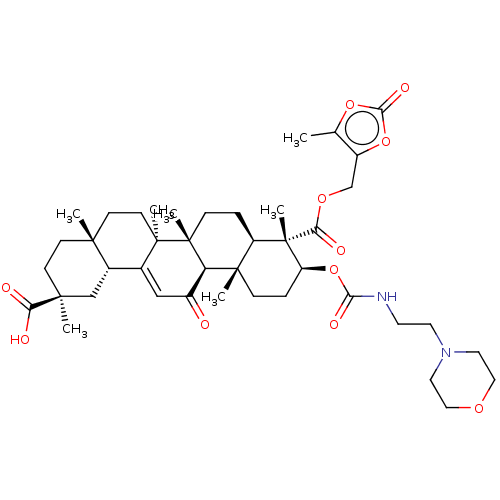 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-2,4a,6a...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642915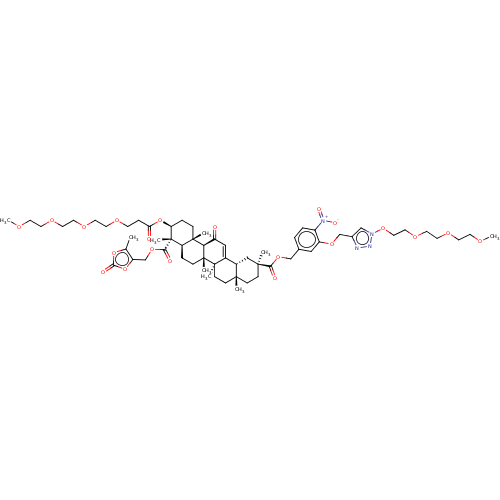 (2-(3-((1-PEG-1H-1,2,3-triazol-4-yl)methoxy)-4-nitr...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642913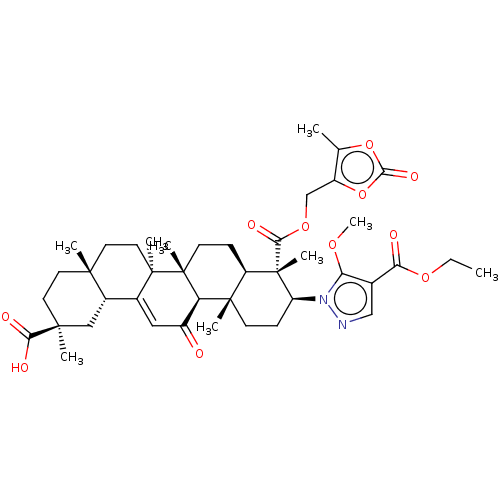 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-10-[4-(...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Mus musculus (mouse)) | BDBM13757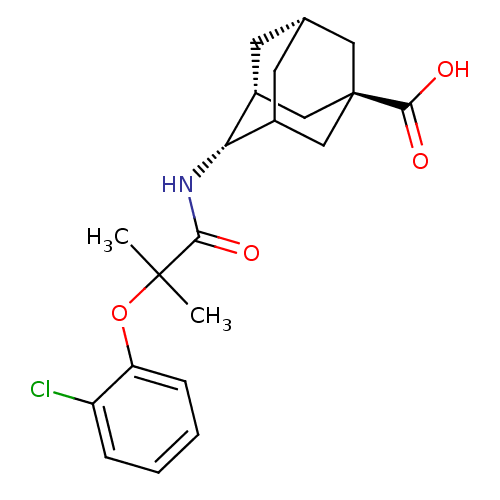 ((1R,3R,4S,7S)-4-[2-(2-chlorophenoxy)-2-methylpropa...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The in Vitro 11beta-HSD2 dehydrogenase assays were developed using the cell lysates that had the full length human or mouse 11beta-HSD2 cDNA over exp... | Bioorg Med Chem Lett 17: 750-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.074 BindingDB Entry DOI: 10.7270/Q2W66J13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM642898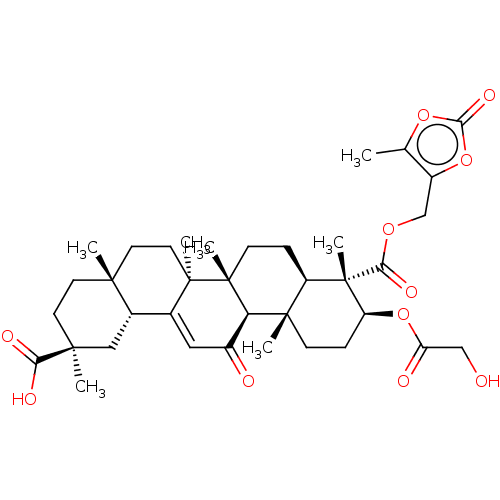 ((2S,4aS,6aS,6bR,8aR,9S,10S,12aS,12bR,14bR)-10-(2-H...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50247012 (3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD2 (unknown origin) expressed in HEK293 cells using cortisone as substrate measured after 2 hrs in presence of NAD+ by HTRF as... | J Med Chem 62: 6925-6940 (2019) Article DOI: 10.1021/acs.jmedchem.9b00187 BindingDB Entry DOI: 10.7270/Q23R0X43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 894 total ) | Next | Last >> |