 Found 756 hits of ic50 for UniProtKB: P00747
Found 756 hits of ic50 for UniProtKB: P00747 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasminogen
(Homo sapiens (Human)) | BDBM50120225

(2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...)Show SMILES CC(O)C(N(C)C(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CCC[N-]C(N)=[NH2+])C(=O)NC(C)c1ccccc1 Show InChI InChI=1S/C27H37N7O6/c1-17(20-8-5-4-6-9-20)31-25(37)24(18(2)35)33(3)26(38)22(32-23(36)10-7-15-30-27(28)29)16-19-11-13-21(14-12-19)34(39)40/h4-6,8-9,11-14,17-18,22,24,35H,7,10,15-16H2,1-3H3,(H6,28,29,30,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit Plasmin was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514073

(CHEMBL4576519)Show SMILES CC(C)NC(=O)c1ccc(c(C)c1)-c1ccc(C[C@H](NC(=O)[C@H]2CC[C@H](CN)CC2)C(=O)Nc2cc(Cl)c3cn[nH]c3c2)cc1 |r,wU:25.26,wD:22.22,18.18,(23.11,-16.8,;23.1,-15.26,;24.43,-14.49,;21.77,-14.5,;20.43,-15.27,;20.44,-16.81,;19.1,-14.5,;17.76,-15.28,;16.43,-14.51,;16.43,-12.97,;17.76,-12.19,;17.75,-10.65,;19.09,-12.96,;15.1,-12.2,;13.76,-12.97,;12.43,-12.2,;12.44,-10.66,;11.11,-9.89,;11.11,-8.35,;9.77,-7.58,;8.44,-8.35,;7.1,-7.58,;8.44,-9.89,;7.11,-10.66,;7.11,-12.2,;8.44,-12.97,;8.44,-14.51,;7.1,-15.28,;9.77,-12.2,;9.77,-10.66,;12.44,-7.58,;12.44,-6.04,;13.77,-8.35,;15.11,-7.58,;15.1,-6.05,;16.43,-5.27,;16.42,-3.73,;17.77,-6.04,;19.23,-5.58,;20.13,-6.82,;19.22,-8.06,;17.76,-7.58,;16.44,-8.35,;13.76,-9.89,;15.09,-10.65,)| Show InChI InChI=1S/C35H41ClN6O3/c1-20(2)39-34(44)26-12-13-28(21(3)14-26)24-8-4-22(5-9-24)15-32(41-33(43)25-10-6-23(18-37)7-11-25)35(45)40-27-16-30(36)29-19-38-42-31(29)17-27/h4-5,8-9,12-14,16-17,19-20,23,25,32H,6-7,10-11,15,18,37H2,1-3H3,(H,38,42)(H,39,44)(H,40,45)(H,41,43)/t23-,25-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514076

(CHEMBL4456691)Show SMILES Cc1cc2[nH]c(nc2cc1-c1ccc(C[C@H](NC(=O)[C@H]2CC[C@H](CN)CC2)C(=O)Nc2cc(Cl)c3[nH]c(=O)[nH]c3c2)cc1)C1CC1 |r,wU:22.24,wD:19.20,15.16,(35.34,-31.42,;36.67,-30.65,;38,-31.42,;39.34,-30.65,;40.81,-31.11,;41.71,-29.85,;40.79,-28.61,;39.33,-29.1,;38,-28.34,;36.67,-29.11,;35.34,-28.34,;34,-29.11,;32.67,-28.34,;32.68,-26.8,;31.34,-26.03,;31.34,-24.49,;30.01,-23.72,;28.68,-24.49,;27.34,-23.72,;28.68,-26.03,;27.35,-26.81,;27.35,-28.35,;28.68,-29.11,;28.68,-30.65,;27.34,-31.42,;30,-28.35,;30,-26.81,;32.68,-23.72,;32.68,-22.18,;34.01,-24.49,;35.34,-23.72,;35.34,-22.19,;36.67,-21.42,;36.66,-19.88,;38.01,-22.18,;39.48,-21.71,;40.38,-22.96,;41.92,-22.97,;39.47,-24.21,;38,-23.72,;36.68,-24.5,;34,-26.03,;35.33,-26.8,;43.25,-29.83,;44.59,-30.59,;44.57,-29.05,)| Show InChI InChI=1S/C35H38ClN7O3/c1-18-12-27-28(40-32(39-27)22-10-11-22)16-25(18)21-6-2-19(3-7-21)13-30(41-33(44)23-8-4-20(17-37)5-9-23)34(45)38-24-14-26(36)31-29(15-24)42-35(46)43-31/h2-3,6-7,12,14-16,20,22-23,30H,4-5,8-11,13,17,37H2,1H3,(H,38,45)(H,39,40)(H,41,44)(H2,42,43,46)/t20-,23-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514062

(CHEMBL4436082)Show SMILES Cc1nc2[nH]c(nc2cc1-c1ccc(C[C@H](NC(=O)[C@H]2CC[C@H](CN)CC2)C(=O)Nc2cc(Cl)c3[nH]c(=O)[nH]c3c2)cc1)C1CC1 |r,wU:22.24,wD:19.20,15.16,(55.88,-32.78,;57.22,-32.01,;58.55,-32.78,;59.89,-32,;61.36,-32.46,;62.26,-31.21,;61.34,-29.97,;59.88,-30.46,;58.54,-29.69,;57.22,-30.47,;55.89,-29.7,;54.55,-30.47,;53.22,-29.7,;53.23,-28.16,;51.89,-27.39,;51.89,-25.85,;50.56,-25.08,;49.23,-25.85,;47.89,-25.08,;49.23,-27.39,;47.9,-28.16,;47.9,-29.7,;49.23,-30.47,;49.23,-32.01,;47.89,-32.78,;50.55,-29.7,;50.55,-28.16,;53.23,-25.08,;53.23,-23.54,;54.56,-25.85,;55.89,-25.08,;55.89,-23.55,;57.22,-22.77,;57.21,-21.23,;58.56,-23.53,;60.03,-23.06,;60.93,-24.32,;62.47,-24.32,;60.02,-25.56,;58.55,-25.08,;57.23,-25.85,;54.55,-27.39,;55.88,-28.15,;63.8,-31.19,;65.14,-31.94,;65.12,-30.4,)| Show InChI InChI=1S/C34H37ClN8O3/c1-17-24(15-27-31(37-17)43-30(39-27)21-10-11-21)20-6-2-18(3-7-20)12-28(40-32(44)22-8-4-19(16-36)5-9-22)33(45)38-23-13-25(35)29-26(14-23)41-34(46)42-29/h2-3,6-7,13-15,19,21-22,28H,4-5,8-12,16,36H2,1H3,(H,38,45)(H,40,44)(H,37,39,43)(H2,41,42,46)/t19-,22-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514063

(CHEMBL4447001)Show SMILES CC(C)c1nc2nc(C)c(cc2[nH]1)-c1ccc(C[C@H](NC(=O)[C@H]2CC[C@H](CN)CC2)C(=O)Nc2ccc(cc2)-c2nnc([nH]2)C(F)(F)C(F)(F)C(O)=O)cc1 |r,wU:25.27,wD:22.23,18.19,(16.63,-38.54,;17.41,-37.21,;18.95,-37.23,;16.65,-35.87,;17.29,-34.47,;16.15,-33.42,;16.14,-31.87,;14.8,-31.11,;14.8,-29.57,;13.48,-31.88,;13.48,-33.42,;14.8,-34.18,;15.11,-35.7,;12.14,-31.11,;10.81,-31.88,;9.48,-31.11,;9.48,-29.57,;8.14,-28.8,;8.14,-27.26,;6.82,-26.49,;5.48,-27.26,;4.15,-26.49,;5.48,-28.8,;4.15,-29.58,;4.15,-31.12,;5.48,-31.88,;5.48,-33.42,;4.15,-34.19,;6.81,-31.12,;6.81,-29.58,;9.48,-26.49,;9.48,-24.95,;10.82,-27.26,;12.15,-26.49,;13.48,-27.27,;14.81,-26.49,;14.82,-24.95,;13.47,-24.19,;12.15,-24.96,;16.14,-24.18,;16.3,-22.65,;17.81,-22.33,;18.58,-23.66,;17.55,-24.8,;20.12,-23.65,;20.9,-24.98,;19.34,-24.98,;20.88,-22.31,;20.11,-20.98,;21.65,-20.98,;22.42,-22.31,;23.2,-23.64,;23.19,-20.97,;10.81,-28.8,;12.14,-29.56,)| Show InChI InChI=1S/C38H41F4N9O4/c1-19(2)30-46-28-17-27(20(3)44-32(28)48-30)23-8-4-21(5-9-23)16-29(47-33(52)25-10-6-22(18-43)7-11-25)34(53)45-26-14-12-24(13-15-26)31-49-35(51-50-31)37(39,40)38(41,42)36(54)55/h4-5,8-9,12-15,17,19,22,25,29H,6-7,10-11,16,18,43H2,1-3H3,(H,45,53)(H,47,52)(H,54,55)(H,44,46,48)(H,49,50,51)/t22-,25-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514065
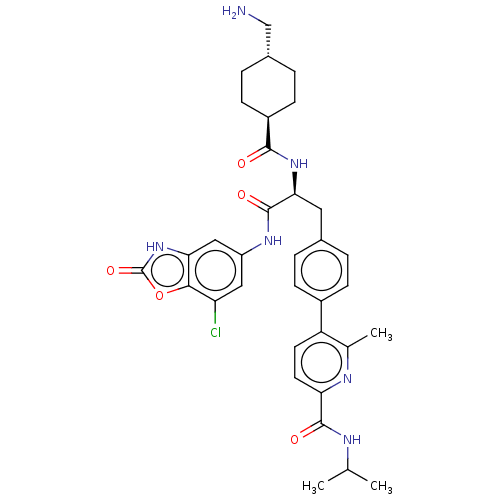
(CHEMBL4570952)Show SMILES CC(C)NC(=O)c1ccc(c(C)n1)-c1ccc(C[C@H](NC(=O)[C@H]2CC[C@H](CN)CC2)C(=O)Nc2cc(Cl)c3oc(=O)[nH]c3c2)cc1 |r,wU:25.26,wD:22.22,18.18,(44.05,-15.78,;44.05,-14.24,;45.38,-13.46,;42.71,-13.47,;41.38,-14.24,;41.38,-15.78,;40.04,-13.48,;38.71,-14.25,;37.38,-13.48,;37.38,-11.94,;38.7,-11.17,;38.7,-9.63,;40.04,-11.93,;36.04,-11.17,;34.71,-11.94,;33.38,-11.17,;33.38,-9.63,;32.05,-8.86,;32.05,-7.32,;30.72,-6.55,;29.38,-7.32,;28.05,-6.55,;29.38,-8.86,;28.05,-9.64,;28.05,-11.18,;29.38,-11.94,;29.38,-13.48,;28.05,-14.25,;30.71,-11.18,;30.71,-9.64,;33.38,-6.55,;33.38,-5.01,;34.72,-7.32,;36.05,-6.55,;36.05,-5.02,;37.37,-4.25,;37.37,-2.71,;38.72,-5.01,;40.19,-4.54,;41.09,-5.79,;42.63,-5.8,;40.17,-7.03,;38.71,-6.55,;37.38,-7.33,;34.71,-8.86,;36.04,-9.62,)| Show InChI InChI=1S/C34H39ClN6O5/c1-18(2)37-32(43)27-13-12-25(19(3)38-27)22-8-4-20(5-9-22)14-29(40-31(42)23-10-6-21(17-36)7-11-23)33(44)39-24-15-26(35)30-28(16-24)41-34(45)46-30/h4-5,8-9,12-13,15-16,18,21,23,29H,6-7,10-11,14,17,36H2,1-3H3,(H,37,43)(H,39,44)(H,40,42)(H,41,45)/t21-,23-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514079
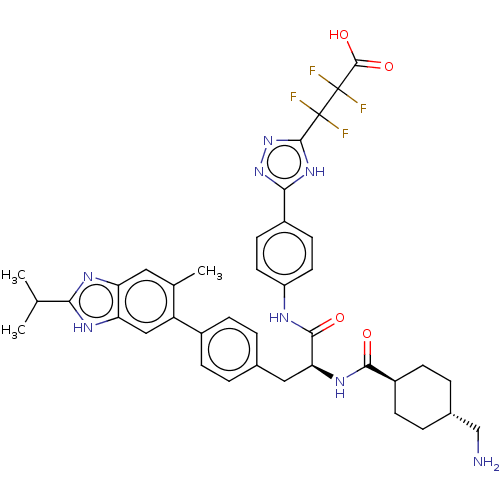
(CHEMBL4449958)Show SMILES CC(C)c1nc2cc(C)c(cc2[nH]1)-c1ccc(C[C@H](NC(=O)[C@H]2CC[C@H](CN)CC2)C(=O)Nc2ccc(cc2)-c2nnc([nH]2)C(F)(F)C(F)(F)C(O)=O)cc1 |r,wU:25.27,wD:22.23,18.19,(59.05,-21.3,;59.83,-19.97,;61.37,-19.99,;59.08,-18.63,;59.72,-17.22,;58.58,-16.18,;58.56,-14.63,;57.23,-13.87,;57.22,-12.33,;55.9,-14.64,;55.9,-16.18,;57.23,-16.94,;57.54,-18.46,;54.57,-13.87,;53.24,-14.64,;51.9,-13.87,;51.91,-12.33,;50.57,-11.56,;50.57,-10.02,;49.24,-9.25,;47.91,-10.02,;46.58,-9.25,;47.91,-11.56,;46.58,-12.34,;46.58,-13.88,;47.91,-14.64,;47.91,-16.18,;46.58,-16.95,;49.24,-13.88,;49.24,-12.34,;51.91,-9.25,;51.91,-7.71,;53.24,-10.02,;54.58,-9.25,;55.91,-10.02,;57.23,-9.25,;57.24,-7.71,;55.9,-6.94,;54.57,-7.72,;58.57,-6.94,;58.73,-5.41,;60.24,-5.08,;61.01,-6.42,;59.98,-7.56,;62.55,-6.41,;63.32,-7.74,;61.77,-7.74,;63.31,-5.07,;62.54,-3.74,;64.07,-3.73,;64.85,-5.06,;65.63,-6.39,;65.62,-3.73,;53.23,-11.56,;54.57,-12.32,)| Show InChI InChI=1S/C39H42F4N8O4/c1-20(2)32-46-29-16-21(3)28(18-30(29)47-32)24-8-4-22(5-9-24)17-31(48-34(52)26-10-6-23(19-44)7-11-26)35(53)45-27-14-12-25(13-15-27)33-49-36(51-50-33)38(40,41)39(42,43)37(54)55/h4-5,8-9,12-16,18,20,23,26,31H,6-7,10-11,17,19,44H2,1-3H3,(H,45,53)(H,46,47)(H,48,52)(H,54,55)(H,49,50,51)/t23-,26-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514077
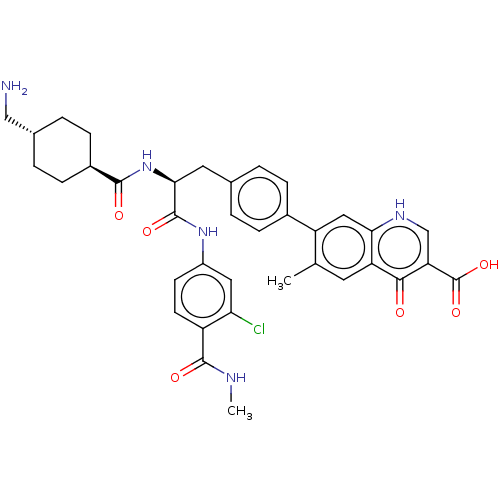
(CHEMBL4475211)Show SMILES CNC(=O)c1ccc(NC(=O)[C@H](Cc2ccc(cc2)-c2cc3[nH]cc(C(O)=O)c(=O)c3cc2C)NC(=O)[C@H]2CC[C@H](CN)CC2)cc1Cl |r,wU:40.43,wD:37.39,11.11,(20.68,-5.12,;19.35,-5.89,;18.02,-5.13,;18.02,-3.59,;16.68,-5.9,;16.68,-7.44,;15.35,-8.22,;14.02,-7.44,;12.68,-8.21,;11.35,-7.44,;11.35,-5.9,;10.01,-8.21,;10.01,-9.75,;11.35,-10.52,;11.34,-12.06,;12.67,-12.83,;14.01,-12.06,;14,-10.51,;12.67,-9.75,;15.34,-12.83,;15.34,-14.37,;16.67,-15.13,;16.67,-16.66,;18,-17.45,;19.33,-16.68,;20.66,-17.46,;22,-16.69,;20.65,-19,;19.35,-15.13,;20.67,-14.37,;18.01,-14.37,;18.01,-12.82,;16.67,-12.06,;16.66,-10.52,;8.69,-7.44,;7.35,-8.21,;6.02,-7.44,;7.35,-9.75,;6.02,-10.52,;6.02,-12.06,;7.35,-12.83,;7.35,-14.37,;6.02,-15.14,;8.68,-12.06,;8.68,-10.52,;14.01,-5.91,;15.34,-5.14,;15.34,-3.6,)| Show InChI InChI=1S/C36H38ClN5O6/c1-19-13-27-30(40-18-28(32(27)43)36(47)48)16-26(19)22-7-3-20(4-8-22)14-31(42-33(44)23-9-5-21(17-38)6-10-23)35(46)41-24-11-12-25(29(37)15-24)34(45)39-2/h3-4,7-8,11-13,15-16,18,21,23,31H,5-6,9-10,14,17,38H2,1-2H3,(H,39,45)(H,40,43)(H,41,46)(H,42,44)(H,47,48)/t21-,23-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514078
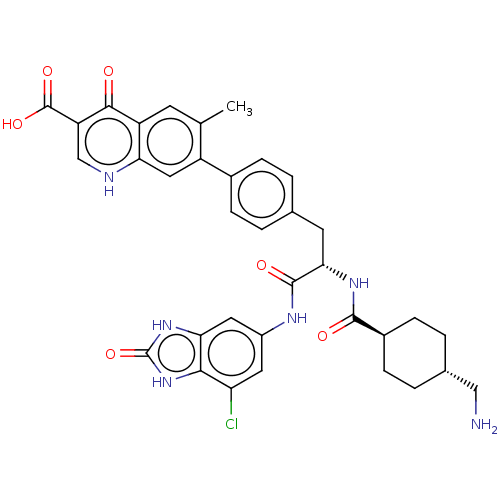
(CHEMBL4582882)Show SMILES Cc1cc2c(cc1-c1ccc(C[C@H](NC(=O)[C@H]3CC[C@H](CN)CC3)C(=O)Nc3cc(Cl)c4[nH]c(=O)[nH]c4c3)cc1)[nH]cc(C(O)=O)c2=O |r,wU:19.20,wD:16.16,12.12,(35.27,-11.33,;35.27,-12.87,;36.61,-13.63,;36.61,-15.18,;35.28,-15.94,;33.95,-15.18,;33.95,-13.64,;32.61,-12.87,;31.28,-13.64,;29.95,-12.87,;29.95,-11.33,;28.61,-10.56,;28.61,-9.02,;27.29,-8.25,;25.95,-9.02,;24.62,-8.25,;25.95,-10.56,;24.62,-11.34,;24.62,-12.88,;25.95,-13.64,;25.95,-15.18,;24.62,-15.95,;27.28,-12.88,;27.28,-11.34,;29.95,-8.25,;29.95,-6.71,;31.29,-9.02,;32.62,-8.25,;32.62,-6.72,;33.94,-5.95,;33.94,-4.41,;35.28,-6.7,;36.76,-6.23,;37.67,-7.49,;39.21,-7.49,;36.75,-8.74,;35.28,-8.25,;33.95,-9.03,;31.28,-10.56,;32.61,-11.33,;35.27,-17.48,;36.6,-18.26,;37.94,-17.49,;39.26,-18.27,;40.6,-17.51,;39.26,-19.81,;37.95,-15.95,;39.28,-15.18,)| Show InChI InChI=1S/C35H35ClN6O6/c1-17-10-24-27(38-16-25(31(24)43)34(46)47)14-23(17)20-6-2-18(3-7-20)11-29(40-32(44)21-8-4-19(15-37)5-9-21)33(45)39-22-12-26(36)30-28(13-22)41-35(48)42-30/h2-3,6-7,10,12-14,16,19,21,29H,4-5,8-9,11,15,37H2,1H3,(H,38,43)(H,39,45)(H,40,44)(H,46,47)(H2,41,42,48)/t19-,21-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 4438
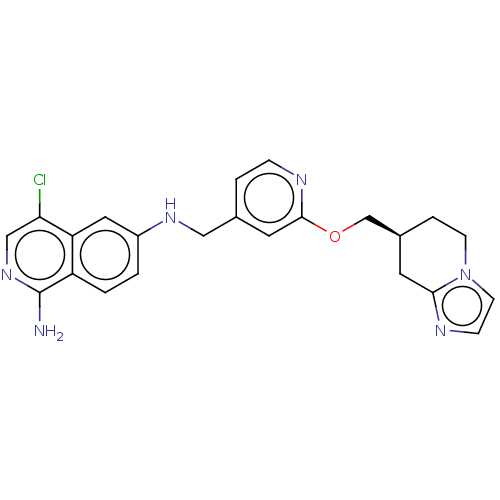
((S*)-4-chloro-N6-((2-((5,6,7,8-tetrahydroimidazo[1...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 4454

(3-chloro-N-((2-((1-methylpiperidin-4-yl)methoxy)py...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 1002

(5-N-({4-[(1-methylpiperidin-4-yl)oxy]phenyl}methyl...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 1128

(5-N-[(6-{5H,6H,8H-imidazo[1,2-a]piperazin-7-yl}pyr...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 1197

(N-((6-(((1-methylpiperidin-4-yl)methyl)amino)pyrid...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 2212

(5-N-[(2-fluoro-4-{2-[(15,4S)-5-isopropyl-2,5-diaza...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 4437
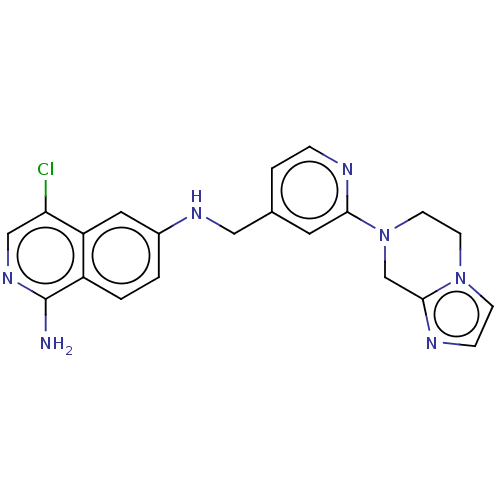
(4-chloro-6-N-[(2-{5H,6H,8H-imidazo[1,2-a]pyrazin-7...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 4308

(4-fluoro-5-N-[(2-{5H,6H,7H,8H-imidazo[1,2-a]pyridi...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 4271
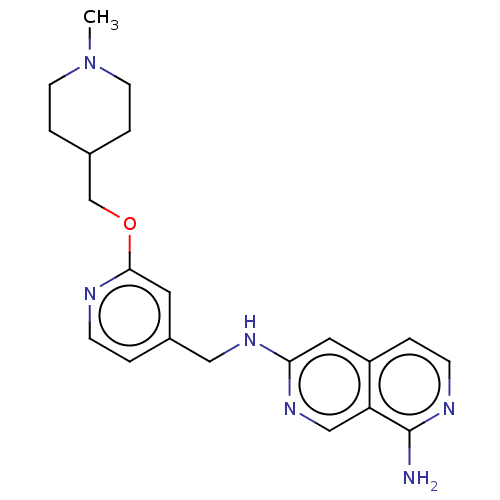
(6-N-({2-[(1-methylpiperidin-4-yl)methoxy]pyridin-4...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 4268
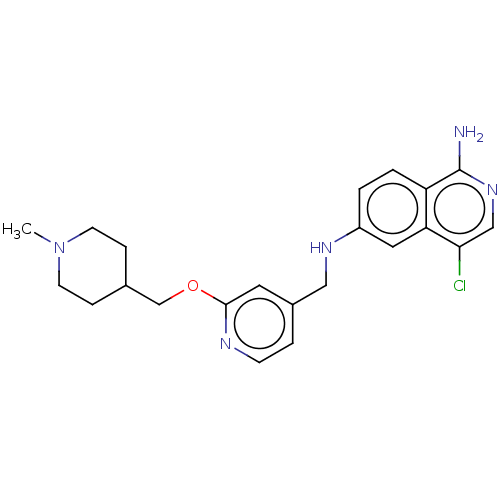
(4-chloro-6-N-({2-[(1-methylpiperidin-4-yl)methoxy]...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 4446
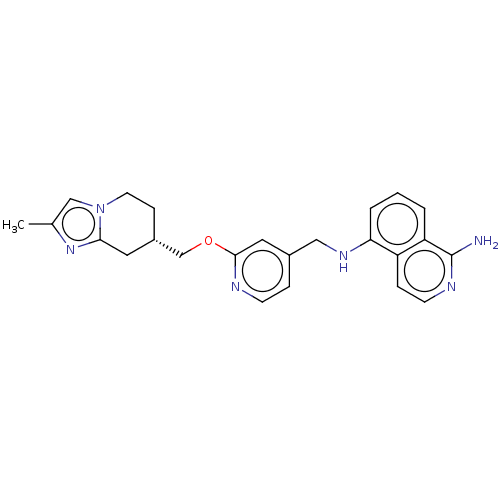
((S*)-N5-((2-((2-methyl-5,6,7,8-tetrahydroimidazo[1...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 4262
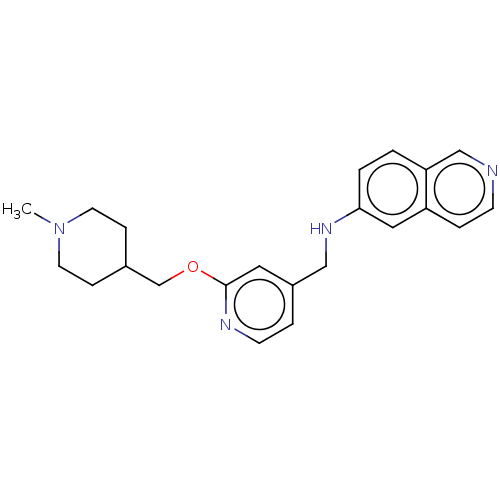
(N-({2-[(1-methylpiperidin-4-yl)methoxy]pyridin-4-y...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 4259

(N6-((2-((1-methylpiperidin-4-yl)oxy)pyridin-4-yl)m...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | US20240059691, Example 1001
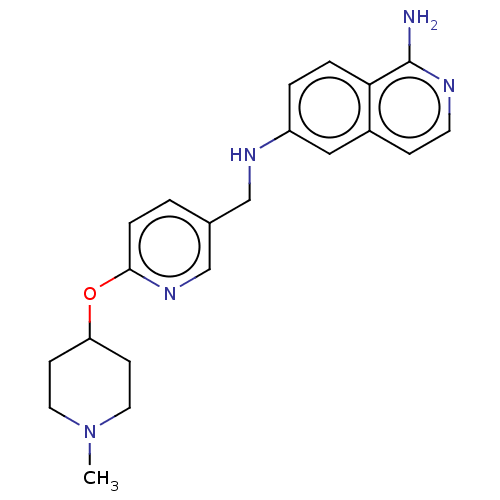
(6-N-({6-[(1-methylpiperidin-4-yl)oxy]pyridin-3-yl}...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514075

(CHEMBL4541084)Show SMILES CCOC(=O)c1ccc(c(C)n1)-c1ccc(C[C@H](NC(=O)[C@H]2CC[C@H](CN)CC2)C(=O)Nc2ccc(cc2)-c2nn[nH]n2)cc1 |r,wU:24.25,wD:21.21,17.17,(24.34,-30.13,;23.01,-30.9,;21.67,-30.14,;20.34,-30.91,;20.35,-32.45,;19.01,-30.15,;17.67,-30.92,;16.34,-30.15,;16.34,-28.61,;17.66,-27.84,;17.66,-26.3,;19,-28.6,;15.01,-27.84,;13.67,-28.61,;12.34,-27.84,;12.35,-26.3,;11.01,-25.53,;11.01,-23.99,;9.68,-23.22,;8.34,-23.99,;7.01,-23.22,;8.34,-25.53,;7.02,-26.3,;7.02,-27.84,;8.34,-28.61,;8.34,-30.15,;7.01,-30.92,;9.67,-27.84,;9.67,-26.3,;12.35,-23.22,;12.35,-21.68,;13.68,-23.99,;15.01,-23.22,;16.34,-23.99,;17.67,-23.22,;17.68,-21.68,;16.34,-20.91,;15.01,-21.69,;19.01,-20.9,;20.42,-21.52,;21.45,-20.38,;20.68,-19.04,;19.17,-19.37,;13.67,-25.53,;15,-26.29,)| Show InChI InChI=1S/C33H38N8O4/c1-3-45-33(44)28-17-16-27(20(2)35-28)23-8-4-21(5-9-23)18-29(37-31(42)25-10-6-22(19-34)7-11-25)32(43)36-26-14-12-24(13-15-26)30-38-40-41-39-30/h4-5,8-9,12-17,22,25,29H,3,6-7,10-11,18-19,34H2,1-2H3,(H,36,43)(H,37,42)(H,38,39,40,41)/t22-,25-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50029498
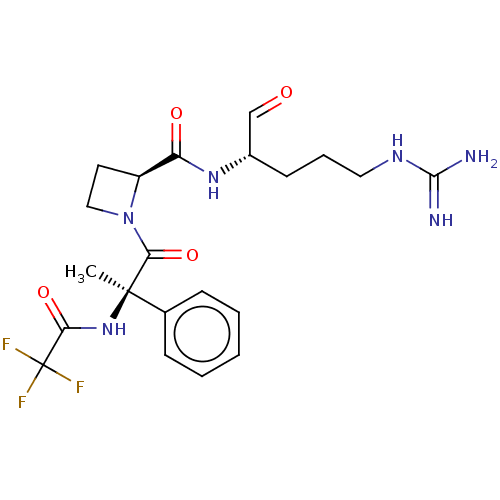
(1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...)Show SMILES [#6][C@]([#7]-[#6](=O)C(F)(F)F)([#6](=O)-[#7]-1-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]=O)c1ccccc1 Show InChI InChI=1S/C21H27F3N6O4/c1-20(13-6-3-2-4-7-13,29-17(33)21(22,23)24)18(34)30-11-9-15(30)16(32)28-14(12-31)8-5-10-27-19(25)26/h2-4,6-7,12,14-15H,5,8-11H2,1H3,(H,28,32)(H,29,33)(H4,25,26,27)/t14-,15-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro Enzyme Inhibitory activity measured against Plasmin |
J Med Chem 38: 4446-53 (1995)
BindingDB Entry DOI: 10.7270/Q2RB73MV |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50124990
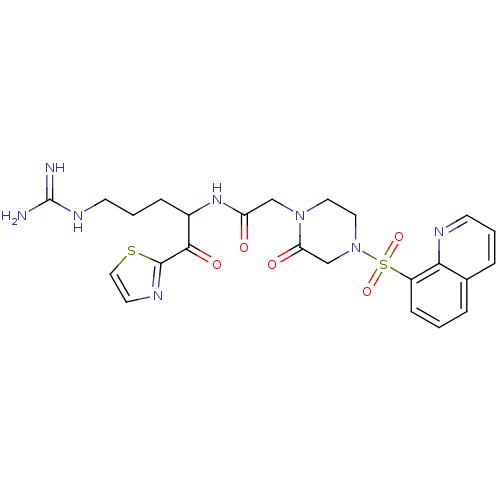
(CHEMBL355376 | N-[4-Guanidino-1-(thiazole-2-carbon...)Show SMILES NC(=N)NCCCC(NC(=O)CN1CCN(CC1=O)S(=O)(=O)c1cccc2cccnc12)C(=O)c1nccs1 Show InChI InChI=1S/C24H28N8O5S2/c25-24(26)29-9-3-6-17(22(35)23-28-10-13-38-23)30-19(33)14-31-11-12-32(15-20(31)34)39(36,37)18-7-1-4-16-5-2-8-27-21(16)18/h1-2,4-5,7-8,10,13,17H,3,6,9,11-12,14-15H2,(H,30,33)(H4,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmin |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50591422
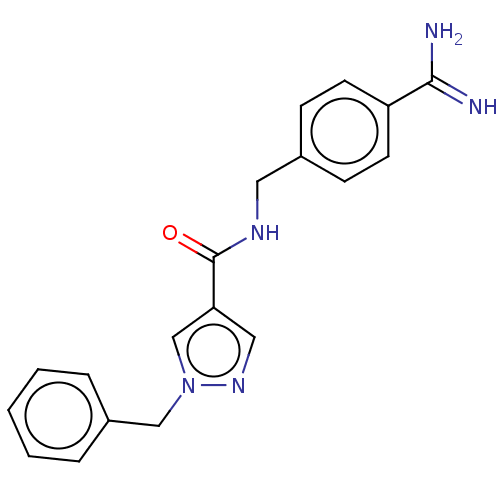
(CHEMBL5175689) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50291006
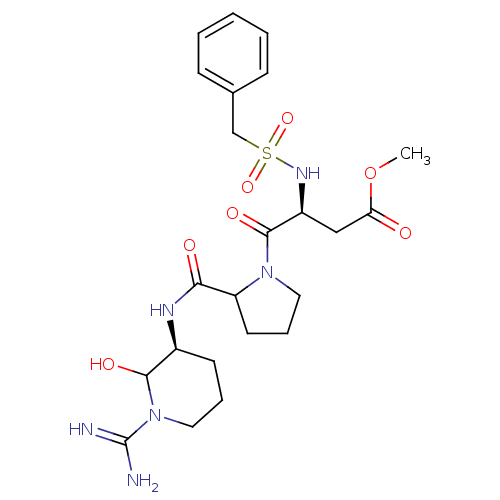
(4-[2-(1-Carbamimidoyl-2-hydroxy-piperidin-3-ylcarb...)Show SMILES COC(=O)C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)N1CCCC1C(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C23H34N6O7S/c1-36-19(30)13-17(27-37(34,35)14-15-7-3-2-4-8-15)22(33)28-11-6-10-18(28)20(31)26-16-9-5-12-29(21(16)32)23(24)25/h2-4,7-8,16-18,21,27,32H,5-6,9-14H2,1H3,(H3,24,25)(H,26,31)/t16-,17-,18?,21?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the inhibitory concentration required to inhibit human serine protease enzyme plasmin by 50% |
Bioorg Med Chem Lett 7: 331-336 (1997)
Article DOI: 10.1016/S0960-894X(97)00004-8
BindingDB Entry DOI: 10.7270/Q2Z03850 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50046356
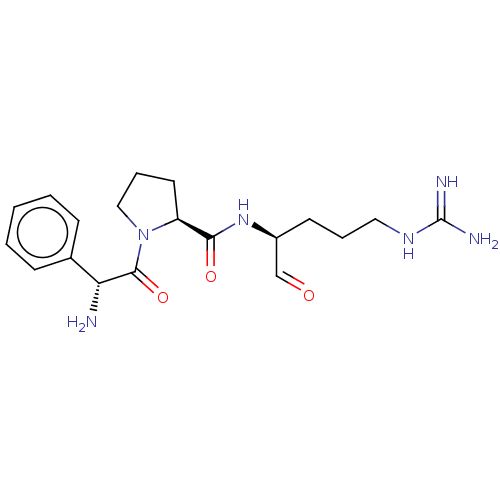
(1-(2-Amino-2-phenyl-acetyl)-pyrrolidine-2-carboxyl...)Show SMILES [#7]-[#6@@H](-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]=O)-c1ccccc1 Show InChI InChI=1S/C19H28N6O3/c20-16(13-6-2-1-3-7-13)18(28)25-11-5-9-15(25)17(27)24-14(12-26)8-4-10-23-19(21)22/h1-3,6-7,12,14-16H,4-5,8-11,20H2,(H,24,27)(H4,21,22,23)/t14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against t-PA (tissue plasminogen activator) |
J Med Chem 36: 314-9 (1993)
BindingDB Entry DOI: 10.7270/Q2930S8J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50046352
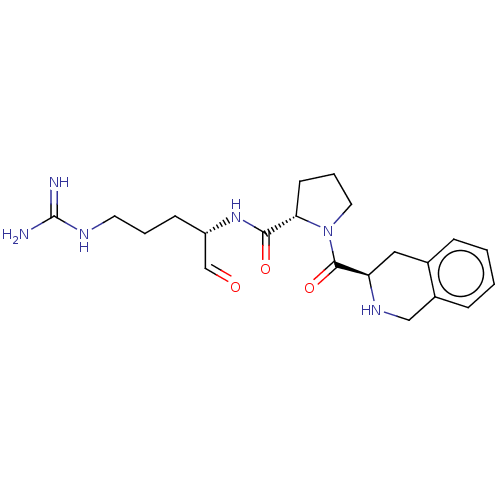
(1-(1,2,3,4-Tetrahydro-isoquinoline-3-carbonyl)-pyr...)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H]1Cc2ccccc2CN1)C=O Show InChI InChI=1S/C21H30N6O3/c22-21(23)24-9-3-7-16(13-28)26-19(29)18-8-4-10-27(18)20(30)17-11-14-5-1-2-6-15(14)12-25-17/h1-2,5-6,13,16-18,25H,3-4,7-12H2,(H,26,29)(H4,22,23,24)/t16-,17+,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against t-PA (tissue plasminogen activator) |
J Med Chem 36: 314-9 (1993)
BindingDB Entry DOI: 10.7270/Q2930S8J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50029507
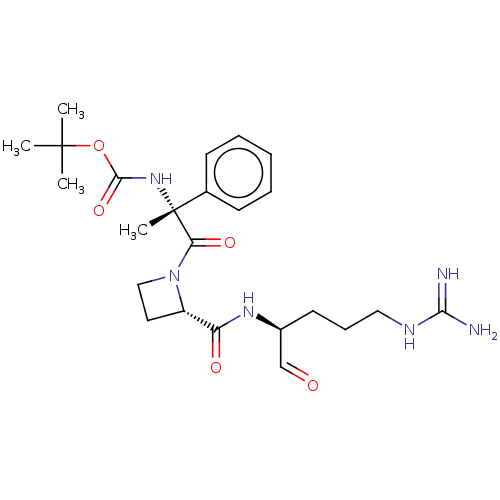
(CHEMBL2370860 | {(R)-2-[2-((S)-1-Formyl-4-guanidin...)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7][C@@]([#6])([#6](=O)-[#7]-1-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]=O)c1ccccc1 Show InChI InChI=1S/C24H36N6O5/c1-23(2,3)35-22(34)29-24(4,16-9-6-5-7-10-16)20(33)30-14-12-18(30)19(32)28-17(15-31)11-8-13-27-21(25)26/h5-7,9-10,15,17-18H,8,11-14H2,1-4H3,(H,28,32)(H,29,34)(H4,25,26,27)/t17-,18-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro Enzyme Inhibitory activity measured against Plasmin |
J Med Chem 38: 4446-53 (1995)
BindingDB Entry DOI: 10.7270/Q2RB73MV |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM200012

(US9234000, 3 | US9512065, N-[(R)-1-[(S)-1-(4-amino...)Show SMILES CCOc1ccc(C[C@@H](NC(=O)c2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)NCc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C35H38N4O4/c1-2-43-30-19-17-26(18-20-30)22-32(38-33(40)29-11-7-4-8-12-29)35(42)39-31(21-25-9-5-3-6-10-25)34(41)37-24-28-15-13-27(23-36)14-16-28/h3-20,31-32H,2,21-24,36H2,1H3,(H,37,41)(H,38,40)(H,39,42)/t31-,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50514074
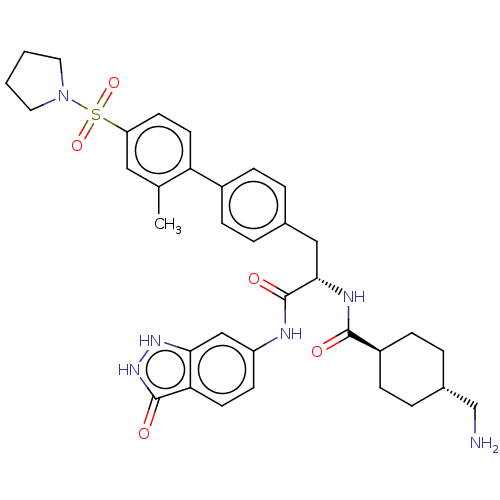
(CHEMBL4539788)Show SMILES Cc1cc(ccc1-c1ccc(C[C@H](NC(=O)[C@H]2CC[C@H](CN)CC2)C(=O)Nc2ccc3c(c2)[nH][nH]c3=O)cc1)S(=O)(=O)N1CCCC1 |r,wU:19.20,wD:16.16,12.12,(63.46,-11.08,;63.46,-12.62,;64.8,-13.38,;64.8,-14.93,;63.47,-15.7,;62.14,-14.94,;62.14,-13.39,;60.8,-12.63,;59.47,-13.4,;58.14,-12.63,;58.14,-11.08,;56.81,-10.31,;56.81,-8.77,;55.48,-8,;54.14,-8.77,;52.81,-8,;54.14,-10.31,;52.81,-11.09,;52.81,-12.63,;54.14,-13.39,;54.14,-14.93,;52.81,-15.7,;55.47,-12.63,;55.47,-11.09,;58.14,-8,;58.14,-6.46,;59.48,-8.77,;60.81,-8,;62.14,-8.78,;63.47,-8,;63.47,-6.46,;62.13,-5.7,;60.81,-6.47,;62.44,-4.19,;63.97,-4.02,;64.61,-5.42,;66.12,-5.73,;59.46,-10.31,;60.8,-11.08,;66.14,-15.7,;66.9,-14.36,;67.68,-15.69,;66.14,-17.24,;64.91,-18.14,;65.39,-19.61,;66.93,-19.6,;67.4,-18.14,)| Show InChI InChI=1S/C35H42N6O5S/c1-22-18-28(47(45,46)41-16-2-3-17-41)13-15-29(22)25-8-4-23(5-9-25)19-32(38-33(42)26-10-6-24(21-36)7-11-26)35(44)37-27-12-14-30-31(20-27)39-40-34(30)43/h4-5,8-9,12-15,18,20,24,26,32H,2-3,6-7,10-11,16-17,19,21,36H2,1H3,(H,37,44)(H,38,42)(H2,39,40,43)/t24-,26-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence... |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228863

((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...)Show SMILES [#6]-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]=O Show InChI InChI=1S/C21H32N6O3/c1-24-17(13-15-7-3-2-4-8-15)20(30)27-12-6-10-18(27)19(29)26-16(14-28)9-5-11-25-21(22)23/h2-4,7-8,14,16-18,24H,5-6,9-13H2,1H3,(H,26,29)(H4,22,23,25)/t16-,17+,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit Plasmin was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM31487

(substituted biphenyl derivative, 36aa)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(cc1C(=O)Nc1ccc(cc1)C(N)=N)-c1ccsc1 Show InChI InChI=1S/C30H28N4O4S/c1-17(2)15-33-28(35)20-6-10-24(26(14-20)30(37)38)23-9-5-19(21-11-12-39-16-21)13-25(23)29(36)34-22-7-3-18(4-8-22)27(31)32/h3-14,16-17H,15H2,1-2H3,(H3,31,32)(H,33,35)(H,34,36)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals
| Assay Description
The assay is an amidolytic assay based upon the absorbance of derivatized thiols from the hydrolysis of Z-Lys-SBzl (Bachem) in the presence of 5,5-Di... |
Bioorg Med Chem 17: 3934-58 (2009)
Article DOI: 10.1016/j.bmc.2009.04.013
BindingDB Entry DOI: 10.7270/Q2SX6BJN |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50374312

(CHEMBL270834)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]S(=O)(=O)[#6]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-c1nc2ccccc2o1 Show InChI InChI=1S/C29H40N10O6S/c30-28(31)34-15-6-11-21(25(41)27-38-20-10-4-5-13-23(20)45-27)37-24(40)18-36-26(42)22(12-7-16-35-29(32)33)39-46(43,44)17-14-19-8-2-1-3-9-19/h1-5,8-10,13,21-22,39H,6-7,11-12,14-18H2,(H,36,42)(H,37,40)(H4,30,31,34)(H4,32,33,35)/t21-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem 16: 1562-95 (2008)
Article DOI: 10.1016/j.bmc.2007.11.015
BindingDB Entry DOI: 10.7270/Q21J9BNH |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50194743
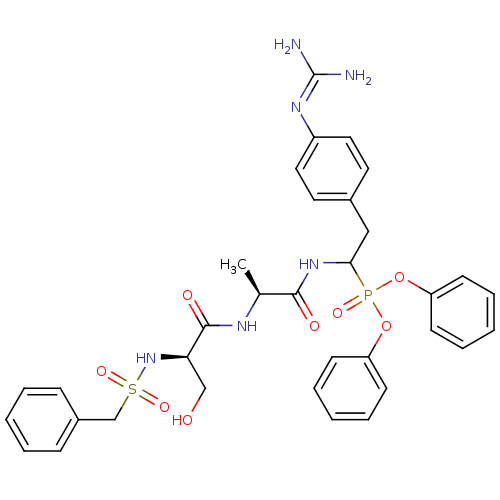
(CHEMBL214814 | diphenyl 1-[(N-alpha-toluenesulfony...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C34H39N6O8PS/c1-24(37-33(43)30(22-41)40-50(45,46)23-26-11-5-2-6-12-26)32(42)39-31(21-25-17-19-27(20-18-25)38-34(35)36)49(44,47-28-13-7-3-8-14-28)48-29-15-9-4-10-16-29/h2-20,24,30-31,40-41H,21-23H2,1H3,(H,37,43)(H,39,42)(H4,35,36,38)/t24-,30+,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 50: 6638-46 (2007)
Article DOI: 10.1021/jm700962j
BindingDB Entry DOI: 10.7270/Q2MW2J0J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50194743

(CHEMBL214814 | diphenyl 1-[(N-alpha-toluenesulfony...)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C34H39N6O8PS/c1-24(37-33(43)30(22-41)40-50(45,46)23-26-11-5-2-6-12-26)32(42)39-31(21-25-17-19-27(20-18-25)38-34(35)36)49(44,47-28-13-7-3-8-14-28)48-29-15-9-4-10-16-29/h2-20,24,30-31,40-41H,21-23H2,1H3,(H,37,43)(H,39,42)(H4,35,36,38)/t24-,30+,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
J Med Chem 49: 5785-93 (2006)
Article DOI: 10.1021/jm060622g
BindingDB Entry DOI: 10.7270/Q2SF2VT3 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50046360

(CHEMBL104472 | {1-Benzyl-2-[2-(1-formyl-4-guanidin...)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]=O Show InChI InChI=1S/C25H38N6O5/c1-25(2,3)36-24(35)30-19(15-17-9-5-4-6-10-17)22(34)31-14-8-12-20(31)21(33)29-18(16-32)11-7-13-28-23(26)27/h4-6,9-10,16,18-20H,7-8,11-15H2,1-3H3,(H,29,33)(H,30,35)(H4,26,27,28)/t18-,19+,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against t-PA (tissue plasminogen activator) |
J Med Chem 36: 314-9 (1993)
BindingDB Entry DOI: 10.7270/Q2930S8J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50046358
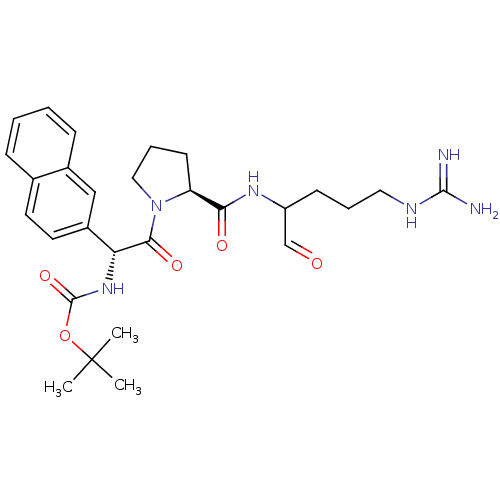
(CHEMBL110780 | {2-[2-(1-Formyl-4-guanidino-butylca...)Show SMILES CC(C)(C)OC(=O)N[C@@H](C(=O)N1CCC[C@H]1C(=O)NC(CCCNC(N)=N)C=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C28H38N6O5/c1-28(2,3)39-27(38)33-23(20-13-12-18-8-4-5-9-19(18)16-20)25(37)34-15-7-11-22(34)24(36)32-21(17-35)10-6-14-31-26(29)30/h4-5,8-9,12-13,16-17,21-23H,6-7,10-11,14-15H2,1-3H3,(H,32,36)(H,33,38)(H4,29,30,31)/t21?,22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasmin |
J Med Chem 36: 314-9 (1993)
BindingDB Entry DOI: 10.7270/Q2930S8J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM31497

(substituted biphenyl derivative, 36ak)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(cc1C(=O)Nc1ccc(cc1)C(N)=N)-c1cscc1CO Show InChI InChI=1S/C31H30N4O5S/c1-17(2)13-34-29(37)20-6-10-24(26(12-20)31(39)40)23-9-5-19(27-16-41-15-21(27)14-36)11-25(23)30(38)35-22-7-3-18(4-8-22)28(32)33/h3-12,15-17,36H,13-14H2,1-2H3,(H3,32,33)(H,34,37)(H,35,38)(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals
| Assay Description
The assay is an amidolytic assay based upon the absorbance of derivatized thiols from the hydrolysis of Z-Lys-SBzl (Bachem) in the presence of 5,5-Di... |
Bioorg Med Chem 17: 3934-58 (2009)
Article DOI: 10.1016/j.bmc.2009.04.013
BindingDB Entry DOI: 10.7270/Q2SX6BJN |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM31467
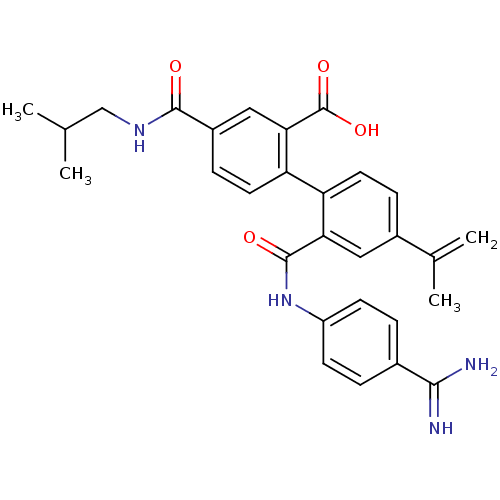
(substituted biphenyl derivative, 36g)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(cc1C(=O)Nc1ccc(cc1)C(N)=N)C(C)=C Show InChI InChI=1S/C29H30N4O4/c1-16(2)15-32-27(34)20-8-12-23(25(14-20)29(36)37)22-11-7-19(17(3)4)13-24(22)28(35)33-21-9-5-18(6-10-21)26(30)31/h5-14,16H,3,15H2,1-2,4H3,(H3,30,31)(H,32,34)(H,33,35)(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals
| Assay Description
The assay is an amidolytic assay based upon the absorbance of derivatized thiols from the hydrolysis of Z-Lys-SBzl (Bachem) in the presence of 5,5-Di... |
Bioorg Med Chem 17: 3934-58 (2009)
Article DOI: 10.1016/j.bmc.2009.04.013
BindingDB Entry DOI: 10.7270/Q2SX6BJN |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50046362
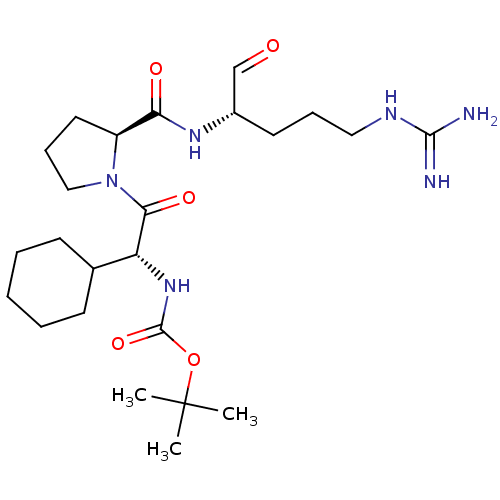
(CHEMBL319930 | {1-Cyclohexyl-2-[2-(1-formyl-4-guan...)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-[#6@H](-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]=O Show InChI InChI=1S/C24H42N6O5/c1-24(2,3)35-23(34)29-19(16-9-5-4-6-10-16)21(33)30-14-8-12-18(30)20(32)28-17(15-31)11-7-13-27-22(25)26/h15-19H,4-14H2,1-3H3,(H,28,32)(H,29,34)(H4,25,26,27)/t17-,18-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasmin |
J Med Chem 36: 314-9 (1993)
BindingDB Entry DOI: 10.7270/Q2930S8J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50046362

(CHEMBL319930 | {1-Cyclohexyl-2-[2-(1-formyl-4-guan...)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-[#6@H](-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]=O Show InChI InChI=1S/C24H42N6O5/c1-24(2,3)35-23(34)29-19(16-9-5-4-6-10-16)21(33)30-14-8-12-18(30)20(32)28-17(15-31)11-7-13-27-22(25)26/h15-19H,4-14H2,1-3H3,(H,28,32)(H,29,34)(H4,25,26,27)/t17-,18-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasmin |
J Med Chem 36: 314-9 (1993)
BindingDB Entry DOI: 10.7270/Q2930S8J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50046372
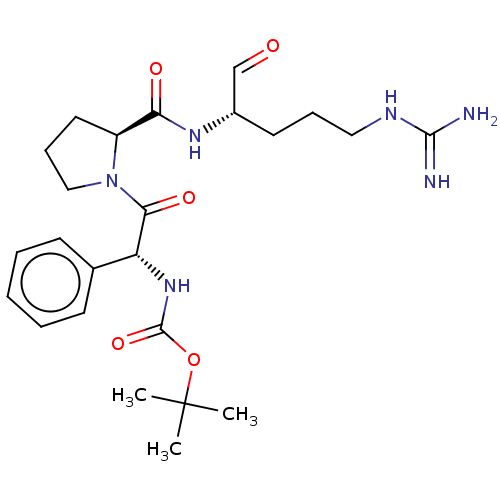
(CHEMBL318998 | {2-[2-(1-Formyl-4-guanidino-butylca...)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-[#6@@H](-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]=O)-c1ccccc1 Show InChI InChI=1S/C24H36N6O5/c1-24(2,3)35-23(34)29-19(16-9-5-4-6-10-16)21(33)30-14-8-12-18(30)20(32)28-17(15-31)11-7-13-27-22(25)26/h4-6,9-10,15,17-19H,7-8,11-14H2,1-3H3,(H,28,32)(H,29,34)(H4,25,26,27)/t17-,18-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against t-PA (tissue plasminogen activator) |
J Med Chem 36: 314-9 (1993)
BindingDB Entry DOI: 10.7270/Q2930S8J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50046372

(CHEMBL318998 | {2-[2-(1-Formyl-4-guanidino-butylca...)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-[#6@@H](-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]=O)-c1ccccc1 Show InChI InChI=1S/C24H36N6O5/c1-24(2,3)35-23(34)29-19(16-9-5-4-6-10-16)21(33)30-14-8-12-18(30)20(32)28-17(15-31)11-7-13-27-22(25)26/h4-6,9-10,15,17-19H,7-8,11-14H2,1-3H3,(H,28,32)(H,29,34)(H4,25,26,27)/t17-,18-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasmin |
J Med Chem 36: 314-9 (1993)
BindingDB Entry DOI: 10.7270/Q2930S8J |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50029506
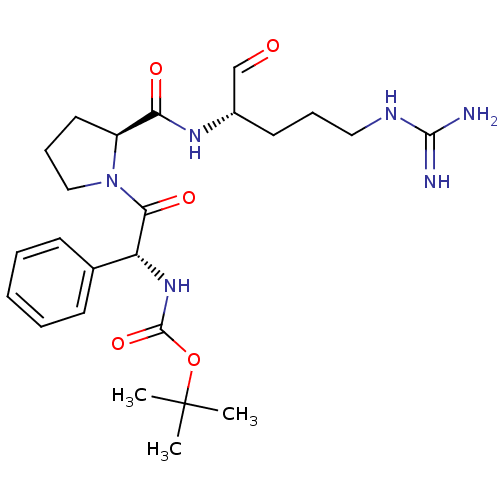
(CHEMBL318998 | tert-butyloxy carbonyl-D-ethylpheny...)Show SMILES CC(C)(C)OC(=O)N[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C=O)c1ccccc1 Show InChI InChI=1S/C24H36N6O5/c1-24(2,3)35-23(34)29-19(16-9-5-4-6-10-16)21(33)30-14-8-12-18(30)20(32)28-17(15-31)11-7-13-27-22(25)26/h4-6,9-10,15,17-19H,7-8,11-14H2,1-3H3,(H,28,32)(H,29,34)(H4,25,26,27)/t17-,18-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro Enzyme Inhibitory activity measured against Plasmin |
J Med Chem 38: 4446-53 (1995)
BindingDB Entry DOI: 10.7270/Q2RB73MV |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50478449
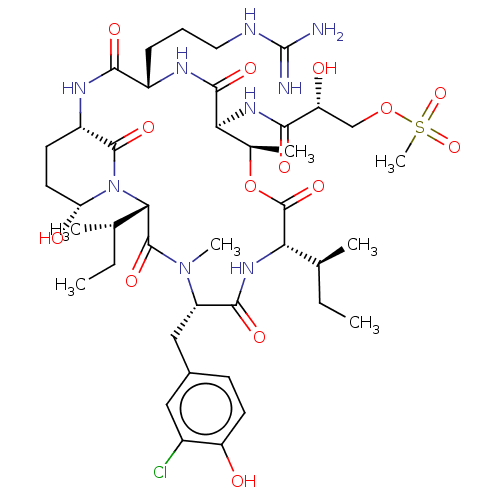
(MICROPEPTIN 478-A | Micropeptin 478 A)Show SMILES [H][C@]12CC[C@@H](O)N(C1=O)[C@@]([H])([C@@H](C)CC)C(=O)N(C)[C@@H](Cc1ccc(O)c(Cl)c1)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)O[C@H](C)[C@H](NC(=O)[C@H](O)COS(C)(=O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C41H64ClN9O14S/c1-8-20(3)31-40(61)65-22(5)32(49-36(57)29(53)19-64-66(7,62)63)37(58)46-25(11-10-16-45-41(43)44)34(55)47-26-13-15-30(54)51(38(26)59)33(21(4)9-2)39(60)50(6)27(35(56)48-31)18-23-12-14-28(52)24(42)17-23/h12,14,17,20-22,25-27,29-33,52-54H,8-11,13,15-16,18-19H2,1-7H3,(H,46,58)(H,47,55)(H,48,56)(H,49,57)(H4,43,44,45)/t20-,21-,22+,25-,26-,27-,29+,30+,31-,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of plasmin after 30 mins by microtiter plate method |
Citation and Details
Article DOI: 10.1021/np9606815
BindingDB Entry DOI: 10.7270/Q27W6FZZ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50124992
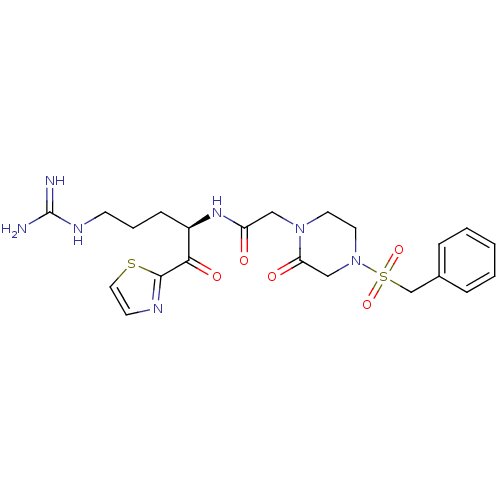
(CHEMBL162461 | N-[(R)-4-Guanidino-1-(thiazole-2-ca...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CN1CCN(CC1=O)S(=O)(=O)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C22H29N7O5S2/c23-22(24)26-8-4-7-17(20(32)21-25-9-12-35-21)27-18(30)13-28-10-11-29(14-19(28)31)36(33,34)15-16-5-2-1-3-6-16/h1-3,5-6,9,12,17H,4,7-8,10-11,13-15H2,(H,27,30)(H4,23,24,26)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plasmin |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50088985
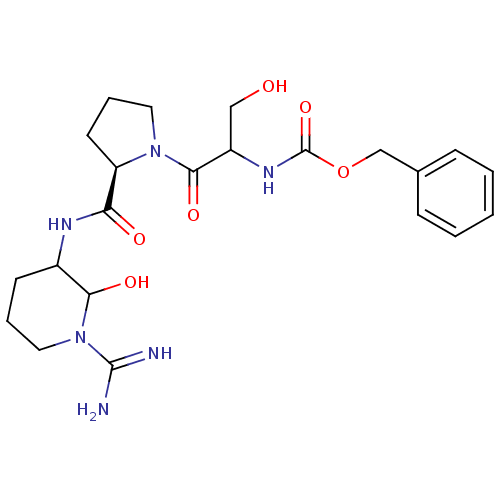
(CHEMBL369042 | {2-[2-(1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES NC(=N)N1CCCC(NC(=O)[C@H]2CCCN2C(=O)C(CO)NC(=O)OCc2ccccc2)C1O Show InChI InChI=1S/C22H32N6O6/c23-21(24)28-11-4-8-15(19(28)31)25-18(30)17-9-5-10-27(17)20(32)16(12-29)26-22(33)34-13-14-6-2-1-3-7-14/h1-3,6-7,15-17,19,29,31H,4-5,8-13H2,(H3,23,24)(H,25,30)(H,26,33)/t15?,16?,17-,19?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 |
Bioorg Med Chem Lett 10: 983-7 (2000)
BindingDB Entry DOI: 10.7270/Q2765DKB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data