 Found 487 hits of ki for UniProtKB: P00750
Found 487 hits of ki for UniProtKB: P00750 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50172853
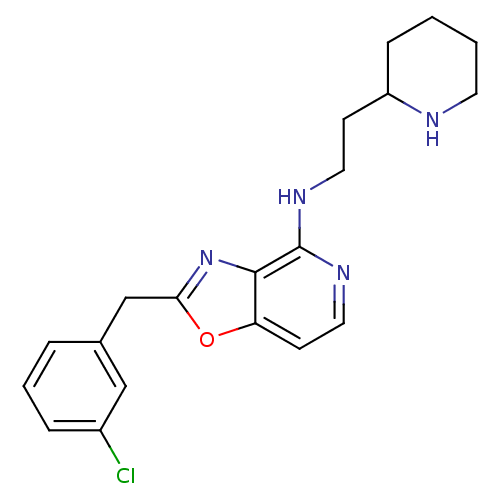
(CHEMBL439678 | [2-(3-Chloro-benzyl)-oxazolo[4,5-c]...)Show InChI InChI=1S/C20H23ClN4O/c21-15-5-3-4-14(12-15)13-18-25-19-17(26-18)8-11-24-20(19)23-10-7-16-6-1-2-9-22-16/h3-5,8,11-12,16,22H,1-2,6-7,9-10,13H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against tissue plasminogen activator |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131480
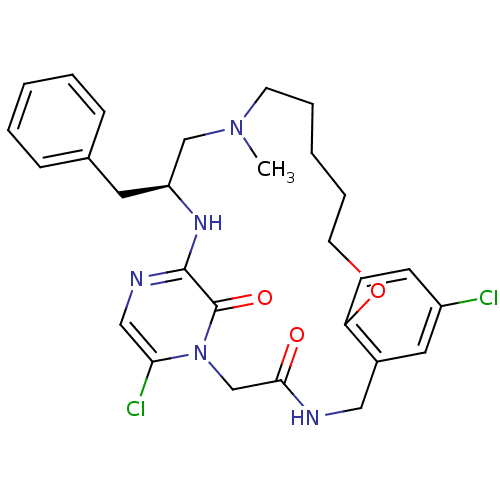
((S)-20-Benzyl-8,25-dichloro-18-methyl-12-oxa-1,4,1...)Show SMILES CN1CCCCCOc2ccc(Cl)cc2CNC(=O)Cn2c(Cl)cnc(N[C@@H](Cc3ccccc3)C1)c2=O Show InChI InChI=1S/C28H33Cl2N5O3/c1-34-12-6-3-7-13-38-24-11-10-22(29)15-21(24)16-31-26(36)19-35-25(30)17-32-27(28(35)37)33-23(18-34)14-20-8-4-2-5-9-20/h2,4-5,8-11,15,17,23H,3,6-7,12-14,16,18-19H2,1H3,(H,31,36)(H,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50288406
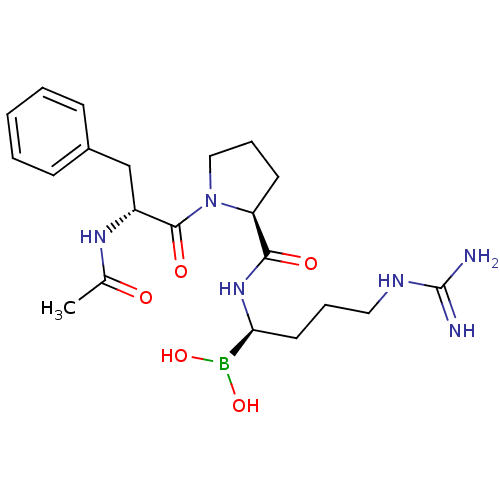
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of tissue plasminogen activator |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131460
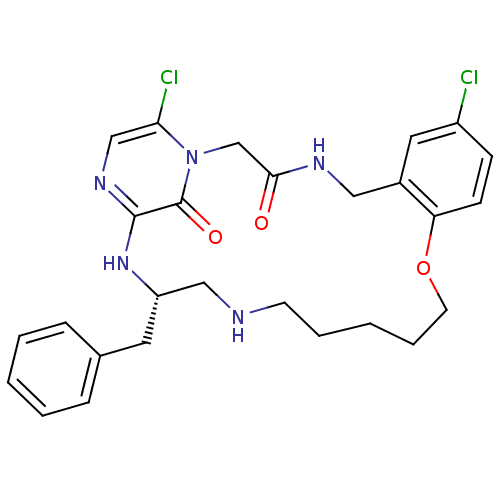
((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,18,21,23-pe...)Show SMILES Clc1ccc2OCCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H31Cl2N5O3/c28-21-9-10-23-20(14-21)15-31-25(35)18-34-24(29)17-32-26(27(34)36)33-22(13-19-7-3-1-4-8-19)16-30-11-5-2-6-12-37-23/h1,3-4,7-10,14,17,22,30H,2,5-6,11-13,15-16,18H2,(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131471
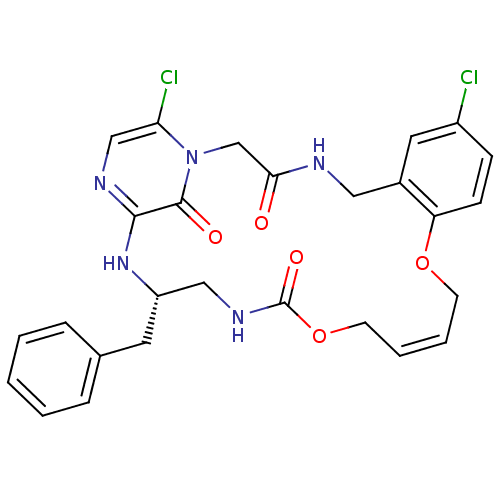
((14Z,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C/COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |c:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4-/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102790

(2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1ccccc1)[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human Serine Protease tissue type Plasminogen Activator (t-PA). |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131466
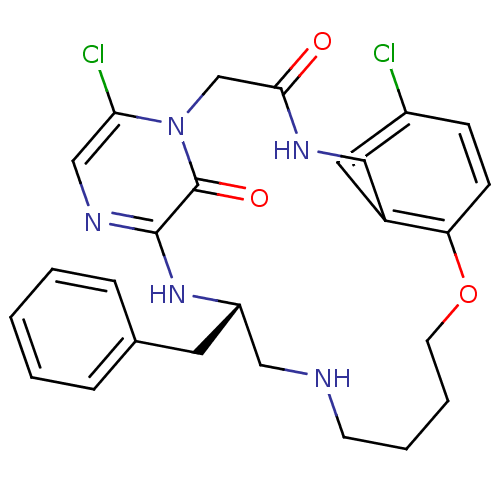
((S)-19-Benzyl-8,24-dichloro-12-oxa-1,4,17,20,22-pe...)Show SMILES Clc1ccc2OCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O3/c27-20-8-9-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-6-2-1-3-7-18)15-29-10-4-5-11-36-22/h1-3,6-9,13,16,21,29H,4-5,10-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115874
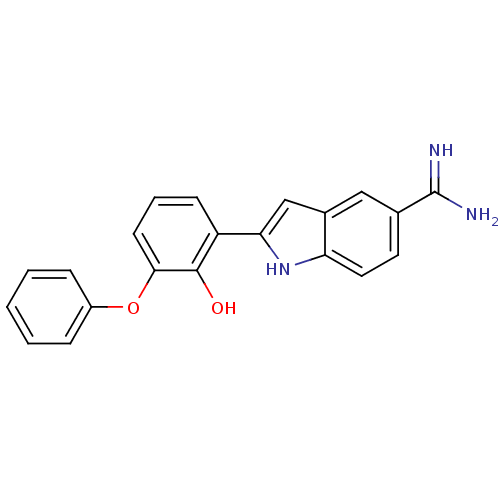
(2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(Oc2ccccc2)c1O Show InChI InChI=1S/C21H17N3O2/c22-21(23)13-9-10-17-14(11-13)12-18(24-17)16-7-4-8-19(20(16)25)26-15-5-2-1-3-6-15/h1-12,24-25H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of tissue-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of human tissue plasminogen activator |
J Med Chem 44: 3856-71 (2001)
BindingDB Entry DOI: 10.7270/Q22R3QXR |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14142
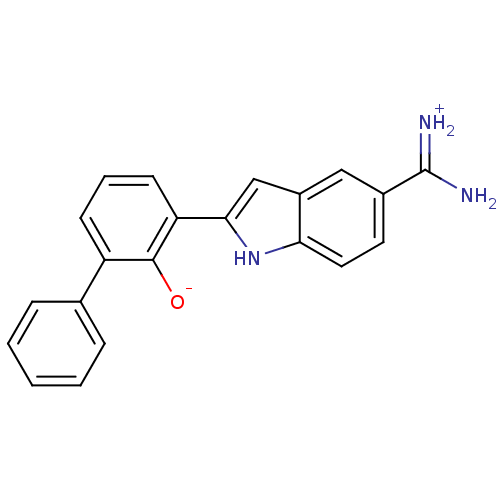
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1[O-] Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50072843
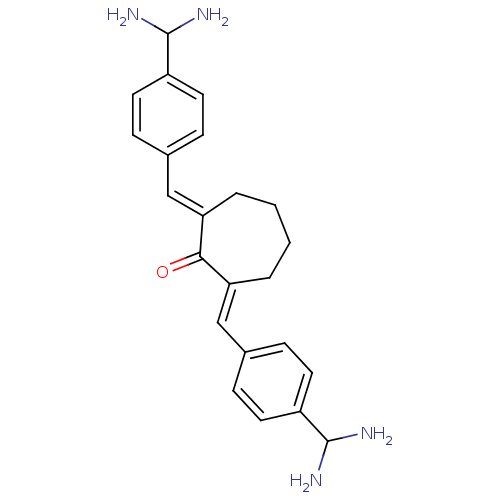
(2,7-Bis-(4-diaminomethyl-benzylidene)-cycloheptano...)Show SMILES NC(N)c1ccc(\C=C2/CCCC\C(=C/c3ccc(cc3)C(N)N)C2=O)cc1 Show InChI InChI=1S/C23H28N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14,22-23H,1-4,24-27H2/b19-13+,20-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue-type plasminogen activator expressed as dissociation constant |
J Med Chem 41: 5445-56 (1999)
Article DOI: 10.1021/jm981068g
BindingDB Entry DOI: 10.7270/Q2KK99ZF |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50288405
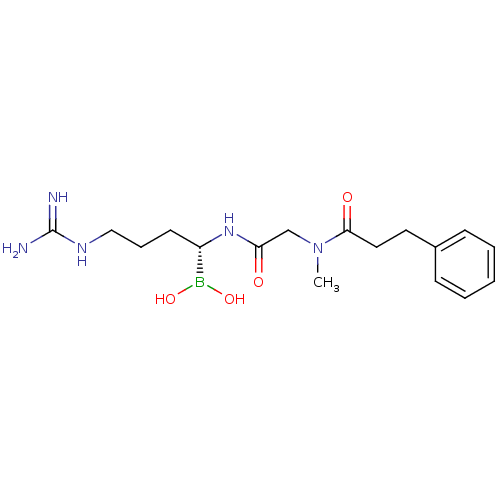
(CHEMBL95940 | N-[(1-Dihydroxyboranyl-4-guanidino-b...)Show SMILES CN(CC(=O)N[C@@H](CCCNC(N)=N)B(O)O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C17H28BN5O4/c1-23(16(25)10-9-13-6-3-2-4-7-13)12-15(24)22-14(18(26)27)8-5-11-21-17(19)20/h2-4,6-7,14,26-27H,5,8-12H2,1H3,(H,22,24)(H4,19,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of tissue plasminogen activator |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50172841
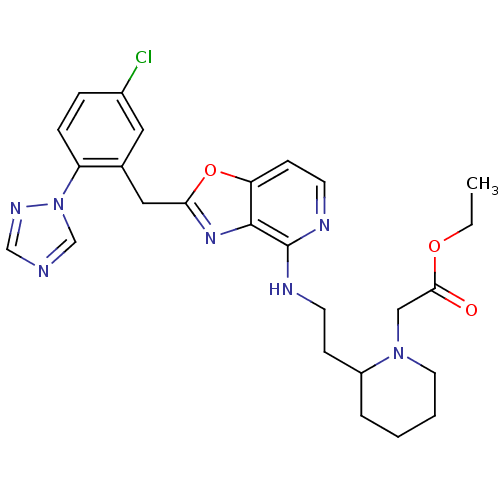
((2-{2-[2-(5-Chloro-2-[1,2,4]triazol-1-yl-benzyl)-o...)Show SMILES CCOC(=O)CN1CCCCC1CCNc1nccc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc12 Show InChI InChI=1S/C26H30ClN7O3/c1-2-36-24(35)15-33-12-4-3-5-20(33)8-10-29-26-25-22(9-11-30-26)37-23(32-25)14-18-13-19(27)6-7-21(18)34-17-28-16-31-34/h6-7,9,11,13,16-17,20H,2-5,8,10,12,14-15H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against tissue plasminogen activator |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102786
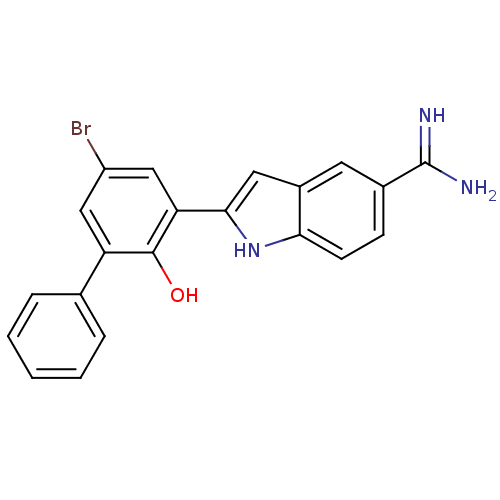
(2-(5-Bromo-2-hydroxy-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(Br)cc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16BrN3O/c22-15-10-16(12-4-2-1-3-5-12)20(26)17(11-15)19-9-14-8-13(21(23)24)6-7-18(14)25-19/h1-11,25-26H,(H3,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50172832
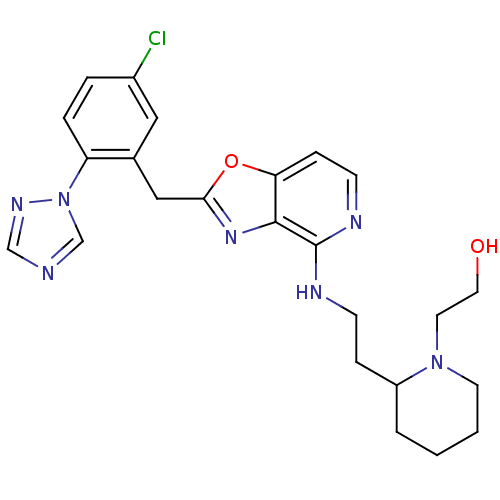
(2-(2-{2-[2-(5-Chloro-2-[1,2,4]triazol-1-yl-benzyl)...)Show SMILES OCCN1CCCCC1CCNc1nccc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc12 Show InChI InChI=1S/C24H28ClN7O2/c25-18-4-5-20(32-16-26-15-29-32)17(13-18)14-22-30-23-21(34-22)7-9-28-24(23)27-8-6-19-3-1-2-10-31(19)11-12-33/h4-5,7,9,13,15-16,19,33H,1-3,6,8,10-12,14H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against tissue plasminogen activator |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50172831
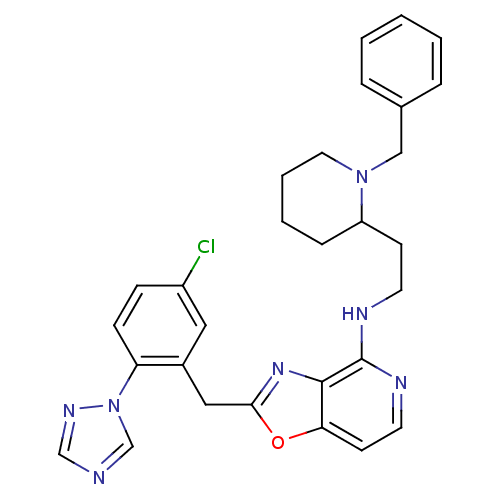
(CHEMBL198978 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES Clc1ccc(c(Cc2nc3c(NCCC4CCCCN4Cc4ccccc4)nccc3o2)c1)-n1cncn1 Show InChI InChI=1S/C29H30ClN7O/c30-23-9-10-25(37-20-31-19-34-37)22(16-23)17-27-35-28-26(38-27)12-14-33-29(28)32-13-11-24-8-4-5-15-36(24)18-21-6-2-1-3-7-21/h1-3,6-7,9-10,12,14,16,19-20,24H,4-5,8,11,13,15,17-18H2,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against tissue plasminogen activator |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of human plasmin |
J Med Chem 44: 3856-71 (2001)
BindingDB Entry DOI: 10.7270/Q22R3QXR |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50076221
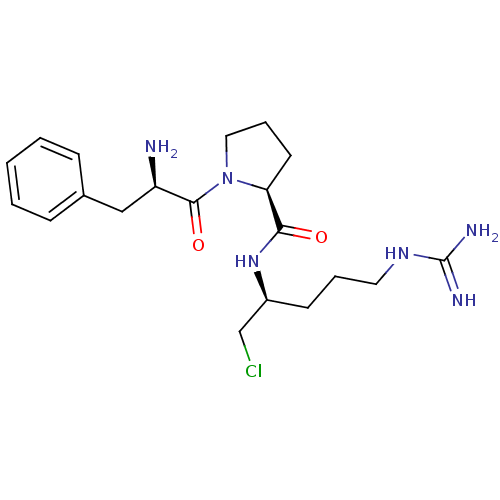
((S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CCl)CCCNC(N)=N Show InChI InChI=1S/C20H31ClN6O2/c21-13-15(8-4-10-25-20(23)24)26-18(28)17-9-5-11-27(17)19(29)16(22)12-14-6-2-1-3-7-14/h1-3,6-7,15-17H,4-5,8-13,22H2,(H,26,28)(H4,23,24,25)/t15-,16+,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Tissue type plasminogen activator (tissue plasminogen activator) |
J Med Chem 42: 1367-75 (1999)
Article DOI: 10.1021/jm980354p
BindingDB Entry DOI: 10.7270/Q20Z72G1 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50172845
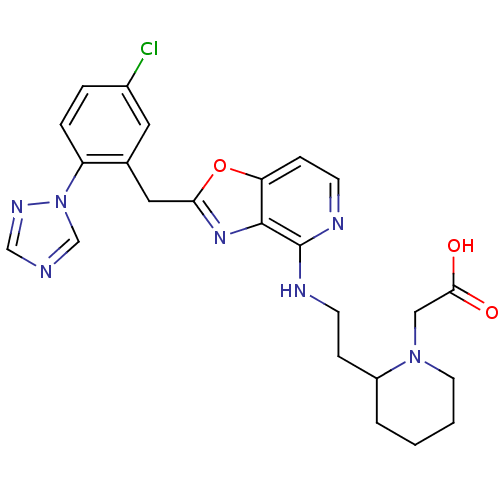
((2-{2-[2-(5-Chloro-2-[1,2,4]triazol-1-yl-benzyl)-o...)Show SMILES OC(=O)CN1CCCCC1CCNc1nccc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc12 Show InChI InChI=1S/C24H26ClN7O3/c25-17-4-5-19(32-15-26-14-29-32)16(11-17)12-21-30-23-20(35-21)7-9-28-24(23)27-8-6-18-3-1-2-10-31(18)13-22(33)34/h4-5,7,9,11,14-15,18H,1-3,6,8,10,12-13H2,(H,27,28)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against tissue plasminogen activator |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50106240
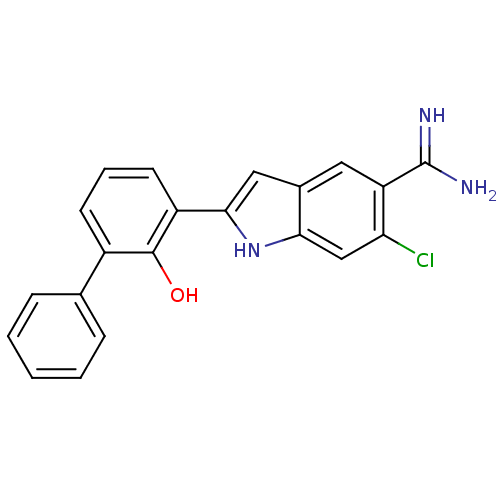
(6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES NC(=N)c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of human plasmin |
J Med Chem 44: 3856-71 (2001)
BindingDB Entry DOI: 10.7270/Q22R3QXR |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101866
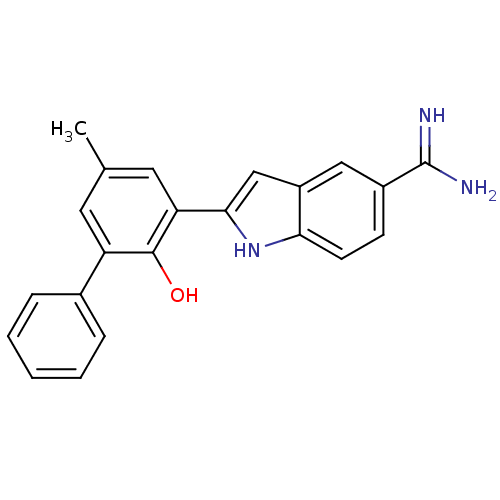
(2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES Cc1cc(-c2cc3cc(ccc3[nH]2)C(N)=N)c(O)c(c1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O/c1-13-9-17(14-5-3-2-4-6-14)21(26)18(10-13)20-12-16-11-15(22(23)24)7-8-19(16)25-20/h2-12,25-26H,1H3,(H3,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50439332
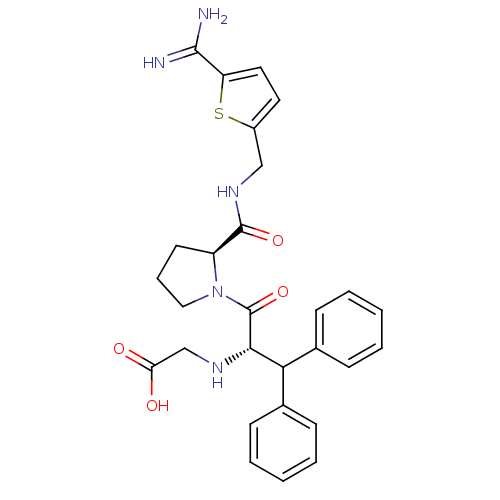
(CHEMBL2419745)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@@H](NCC(O)=O)C(c2ccccc2)c2ccccc2)s1 |r| Show InChI InChI=1S/C28H31N5O4S/c29-26(30)22-14-13-20(38-22)16-32-27(36)21-12-7-15-33(21)28(37)25(31-17-23(34)35)24(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-6,8-11,13-14,21,24-25,31H,7,12,15-17H2,(H3,29,30)(H,32,36)(H,34,35)/t21-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human t-PA |
Bioorg Med Chem Lett 23: 4779-84 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.008
BindingDB Entry DOI: 10.7270/Q2X92CR8 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50003738
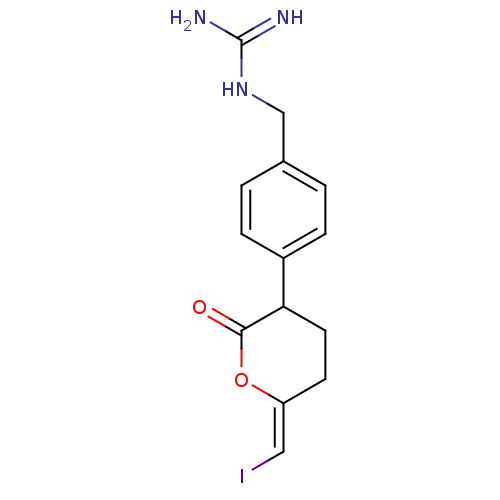
((lactone 2)N-[4-(6-Iodomethylene-2-oxo-tetrahydro-...)Show InChI InChI=1S/C14H16IN3O2/c15-7-11-5-6-12(13(19)20-11)10-3-1-9(2-4-10)8-18-14(16)17/h1-4,7,12H,5-6,8H2,(H4,16,17,18)/b11-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against the enzyme Tissue plasminogen activator |
J Med Chem 35: 4297-305 (1992)
BindingDB Entry DOI: 10.7270/Q2TD9W9D |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM108110

(US8598206, Table 6, 19)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CCC2CCNCC2)NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C35H46N6O4S/c36-33(37)30-17-14-28(15-18-30)24-39-34(42)31(19-16-27-20-22-38-23-21-27)40-35(43)32(13-7-12-26-8-3-1-4-9-26)41-46(44,45)25-29-10-5-2-6-11-29/h1-6,8-11,14-15,17-18,27,31-32,38,41H,7,12-13,16,19-25H2,(H3,36,37)(H,39,42)(H,40,43)/t31-,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human tissue-type plasminogen activator using Mes-d-Cha- Gly-Arg-pNA as substrate |
J Med Chem 63: 1445-1472 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01060
BindingDB Entry DOI: 10.7270/Q2VQ361P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147089
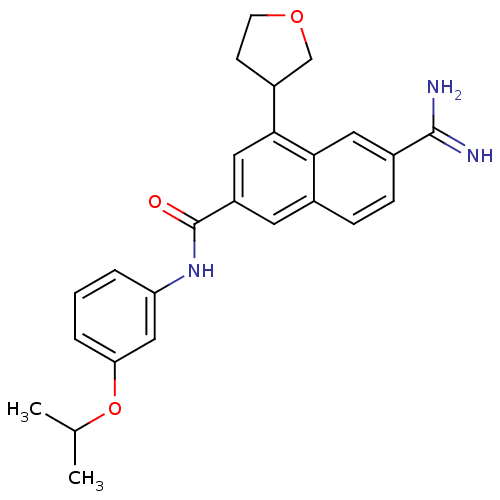
(6-Carbamimidoyl-4-(tetrahydro-furan-3-yl)-naphthal...)Show SMILES CC(C)Oc1cccc(NC(=O)c2cc(C3CCOC3)c3cc(ccc3c2)C(N)=N)c1 Show InChI InChI=1S/C25H27N3O3/c1-15(2)31-21-5-3-4-20(13-21)28-25(29)19-10-16-6-7-17(24(26)27)11-22(16)23(12-19)18-8-9-30-14-18/h3-7,10-13,15,18H,8-9,14H2,1-2H3,(H3,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 3063-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.030
BindingDB Entry DOI: 10.7270/Q29K49PG |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50172826
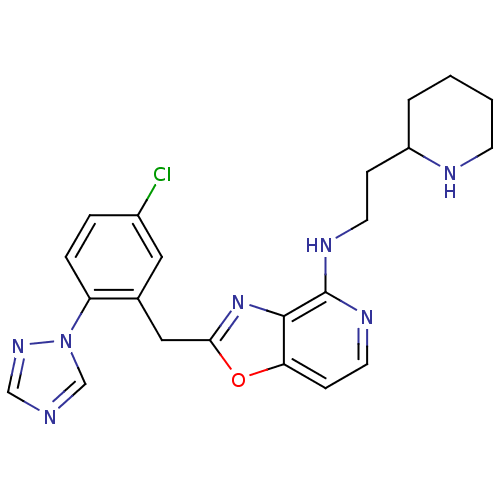
(CHEMBL426101 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES Clc1ccc(c(Cc2nc3c(NCCC4CCCCN4)nccc3o2)c1)-n1cncn1 Show InChI InChI=1S/C22H24ClN7O/c23-16-4-5-18(30-14-24-13-28-30)15(11-16)12-20-29-21-19(31-20)7-10-27-22(21)26-9-6-17-3-1-2-8-25-17/h4-5,7,10-11,13-14,17,25H,1-3,6,8-9,12H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against tissue plasminogen activator |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131464
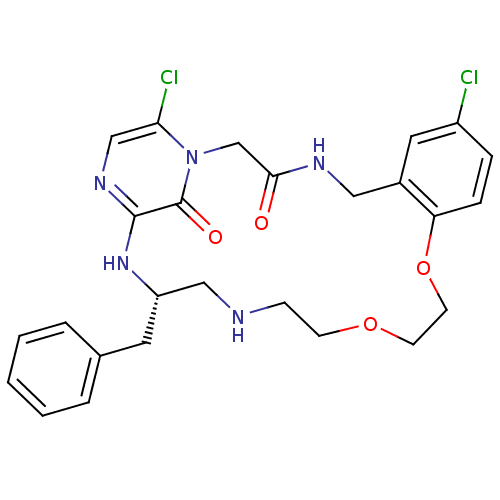
((S)-20-Benzyl-8,25-dichloro-12,15-dioxa-1,4,18,21,...)Show SMILES Clc1ccc2OCCOCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O4/c27-20-6-7-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-4-2-1-3-5-18)15-29-8-9-36-10-11-37-22/h1-7,13,16,21,29H,8-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102778
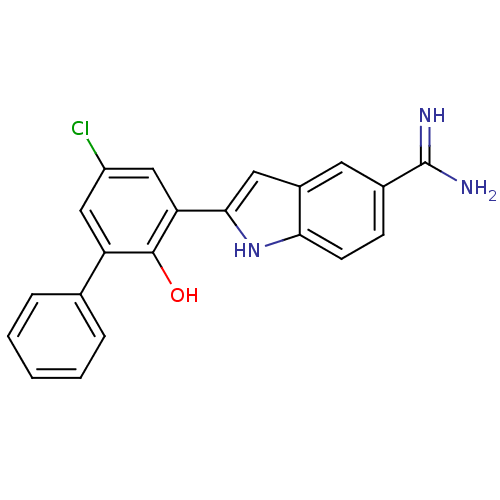
(2-(5-Chloro-2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(Cl)cc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-15-10-16(12-4-2-1-3-5-12)20(26)17(11-15)19-9-14-8-13(21(23)24)6-7-18(14)25-19/h1-11,25-26H,(H3,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14143
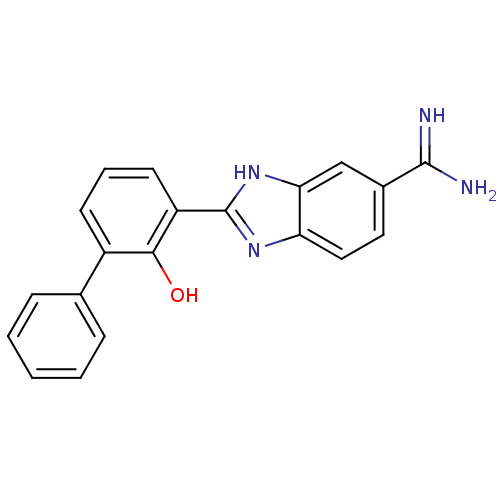
(2-(2-HYDROXY-BIPHENYL)-1H-BENZOIMIDAZOLE-5-CARBOXA...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C20H16N4O/c21-19(22)13-9-10-16-17(11-13)24-20(23-16)15-8-4-7-14(18(15)25)12-5-2-1-3-6-12/h1-11,25H,(H3,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147088
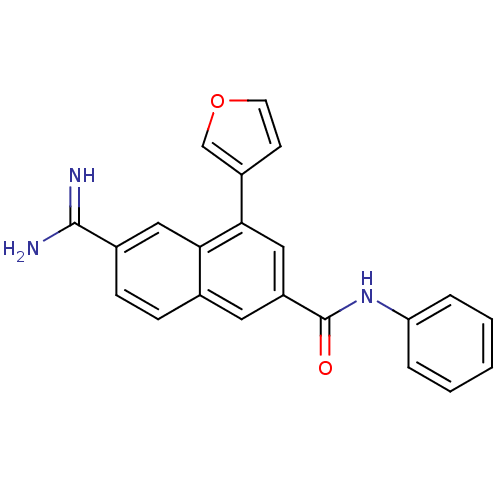
(6-Carbamimidoyl-4-furan-3-yl-naphthalene-2-carboxy...)Show SMILES NC(=N)c1ccc2cc(cc(-c3ccoc3)c2c1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C22H17N3O2/c23-21(24)15-7-6-14-10-17(22(26)25-18-4-2-1-3-5-18)12-20(19(14)11-15)16-8-9-27-13-16/h1-13H,(H3,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity value against Tissue plasminogen activator |
Bioorg Med Chem Lett 15: 93-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.026
BindingDB Entry DOI: 10.7270/Q2R210W5 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147088

(6-Carbamimidoyl-4-furan-3-yl-naphthalene-2-carboxy...)Show SMILES NC(=N)c1ccc2cc(cc(-c3ccoc3)c2c1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C22H17N3O2/c23-21(24)15-7-6-14-10-17(22(26)25-18-4-2-1-3-5-18)12-20(19(14)11-15)16-8-9-27-13-16/h1-13H,(H3,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 3063-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.030
BindingDB Entry DOI: 10.7270/Q29K49PG |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14157

(APC-10605 | CHEMBL64097 | N-(4-carbamimidoylphenyl...)Show InChI InChI=1S/C15H14IN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50106243

(2-(2-Hydroxy-biphenyl-3-yl)-6-methyl-1H-indole-5-c...)Show SMILES Cc1cc2[nH]c(cc2cc1C(N)=N)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C22H19N3O/c1-13-10-19-15(11-18(13)22(23)24)12-20(25-19)17-9-5-8-16(21(17)26)14-6-3-2-4-7-14/h2-12,25-26H,1H3,(H3,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of human plasmin |
J Med Chem 44: 3856-71 (2001)
BindingDB Entry DOI: 10.7270/Q22R3QXR |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14157

(APC-10605 | CHEMBL64097 | N-(4-carbamimidoylphenyl...)Show InChI InChI=1S/C15H14IN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Activation of plasminogen |
Bioorg Med Chem Lett 12: 2023-6 (2002)
BindingDB Entry DOI: 10.7270/Q2ZG6RK4 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50172829
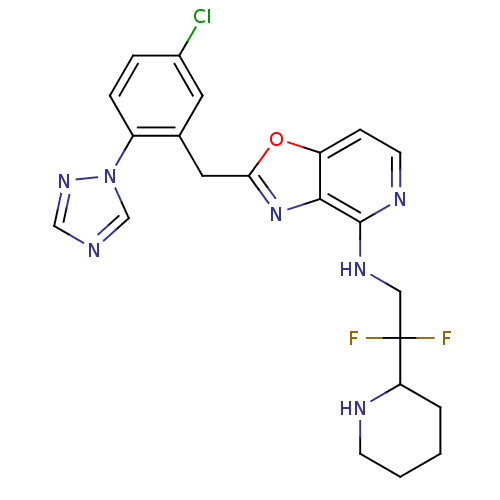
(CHEMBL198735 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES FC(F)(CNc1nccc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc12)C1CCCCN1 Show InChI InChI=1S/C22H22ClF2N7O/c23-15-4-5-16(32-13-26-12-30-32)14(9-15)10-19-31-20-17(33-19)6-8-28-21(20)29-11-22(24,25)18-3-1-2-7-27-18/h4-6,8-9,12-13,18,27H,1-3,7,10-11H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against tissue plasminogen activator |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50288413
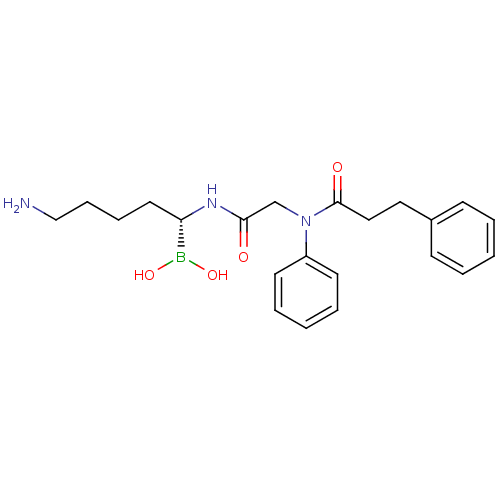
(CHEMBL101759 | N-[(5-Amino-1-dihydroxyboranyl-pent...)Show SMILES NCCCC[C@H](NC(=O)CN(C(=O)CCc1ccccc1)c1ccccc1)B(O)O Show InChI InChI=1S/C22H30BN3O4/c24-16-8-7-13-20(23(29)30)25-21(27)17-26(19-11-5-2-6-12-19)22(28)15-14-18-9-3-1-4-10-18/h1-6,9-12,20,29-30H,7-8,13-17,24H2,(H,25,27)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of tissue plasminogen activator |
Bioorg Med Chem Lett 6: 2913-2918 (1996)
Article DOI: 10.1016/S0960-894X(96)00525-2
BindingDB Entry DOI: 10.7270/Q2V40V6S |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102791
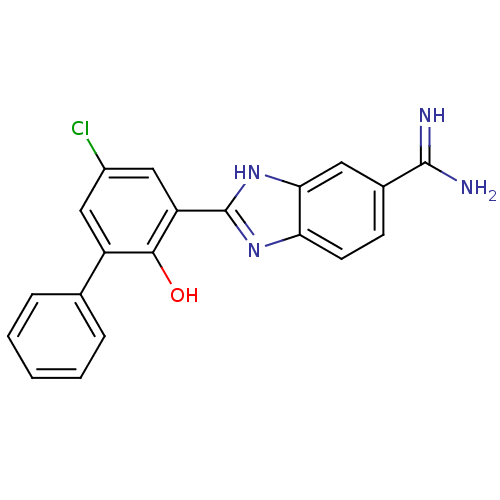
(2-(5-Chloro-2-hydroxy-biphenyl-3-yl)-1H-benzoimida...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2ccccc2)c1O Show InChI InChI=1S/C20H15ClN4O/c21-13-9-14(11-4-2-1-3-5-11)18(26)15(10-13)20-24-16-7-6-12(19(22)23)8-17(16)25-20/h1-10,26H,(H3,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50172830
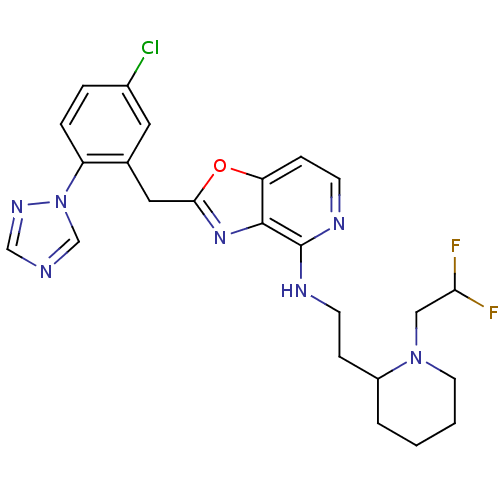
(CHEMBL370396 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES FC(F)CN1CCCCC1CCNc1nccc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc12 Show InChI InChI=1S/C24H26ClF2N7O/c25-17-4-5-19(34-15-28-14-31-34)16(11-17)12-22-32-23-20(35-22)7-9-30-24(23)29-8-6-18-3-1-2-10-33(18)13-21(26)27/h4-5,7,9,11,14-15,18,21H,1-3,6,8,10,12-13H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against tissue plasminogen activator |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14156
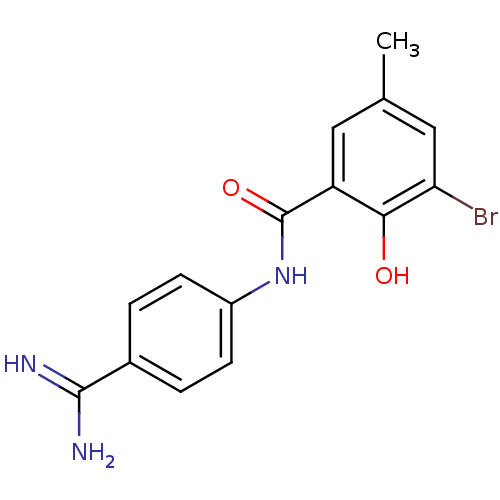
(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14146

(APC-9008 | {amino[2-(3,5-dichloro-2-hydroxyphenyl)...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cc(Cl)cc(Cl)c1O Show InChI InChI=1S/C15H11Cl2N3O/c16-9-5-10(14(21)11(17)6-9)13-4-8-3-7(15(18)19)1-2-12(8)20-13/h1-6,20-21H,(H3,18,19)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50106239

(6-Fluoro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES NC(=N)c1cc2cc([nH]c2cc1F)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16FN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of human plasmin |
J Med Chem 44: 3856-71 (2001)
BindingDB Entry DOI: 10.7270/Q22R3QXR |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131462
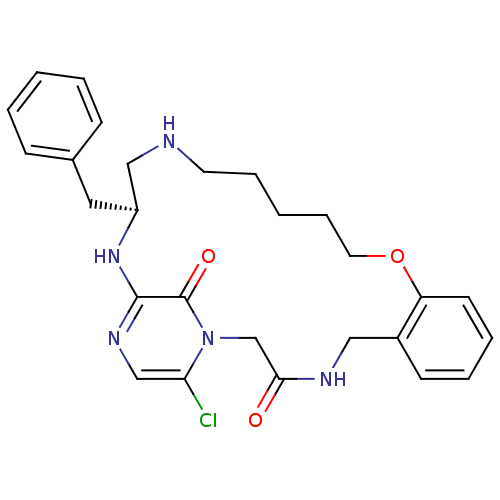
((S)-20-Benzyl-25-chloro-12-oxa-1,4,18,21,23-pentaa...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCCCCOc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C27H32ClN5O3/c28-24-18-31-26-27(35)33(24)19-25(34)30-16-21-11-5-6-12-23(21)36-14-8-2-7-13-29-17-22(32-26)15-20-9-3-1-4-10-20/h1,3-6,9-12,18,22,29H,2,7-8,13-17,19H2,(H,30,34)(H,31,32)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Activation of plasminogen |
Bioorg Med Chem Lett 12: 2023-6 (2002)
BindingDB Entry DOI: 10.7270/Q2ZG6RK4 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14059
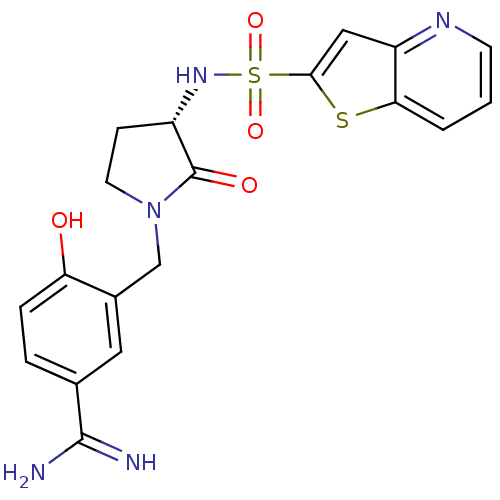
(4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-3-4-15(25)12(8-11)10-24-7-5-13(19(24)26)23-30(27,28)17-9-14-16(29-17)2-1-6-22-14/h1-4,6,8-9,13,23,25H,5,7,10H2,(H3,20,21)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against tissue plasminogen activator (t-Pa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102767

(2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...)Show InChI InChI=1S/C15H12BrN3O/c16-11-3-1-2-10(14(11)20)13-7-9-6-8(15(17)18)4-5-12(9)19-13/h1-7,19-20H,(H3,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131780
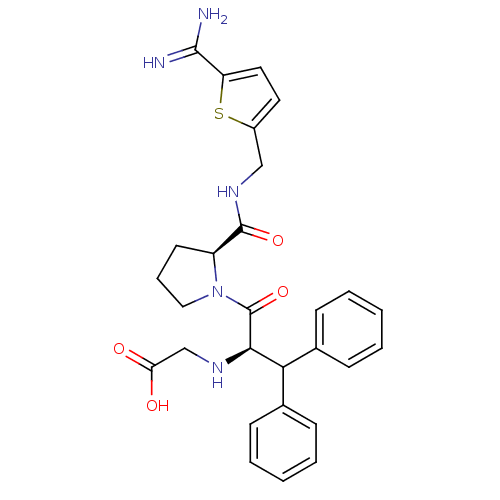
((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NCC(O)=O)C(c2ccccc2)c2ccccc2)s1 Show InChI InChI=1S/C28H31N5O4S/c29-26(30)22-14-13-20(38-22)16-32-27(36)21-12-7-15-33(21)28(37)25(31-17-23(34)35)24(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-6,8-11,13-14,21,24-25,31H,7,12,15-17H2,(H3,29,30)(H,32,36)(H,34,35)/t21-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 433 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human Tissue type plasminogen activator was determined |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101873
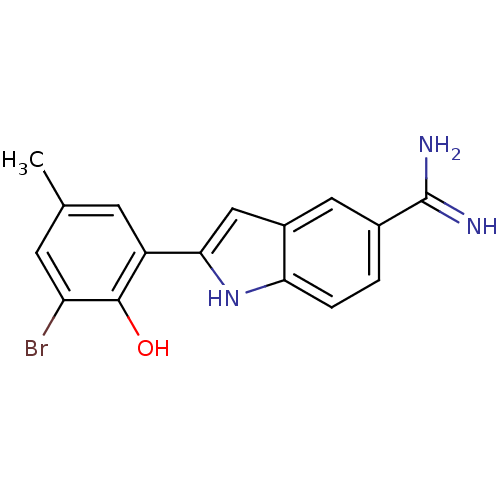
(2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N Show InChI InChI=1S/C16H14BrN3O/c1-8-4-11(15(21)12(17)5-8)14-7-10-6-9(16(18)19)2-3-13(10)20-14/h2-7,20-21H,1H3,(H3,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human Serine Protease tissue type Plasminogen Activator (t-PA). |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50131458
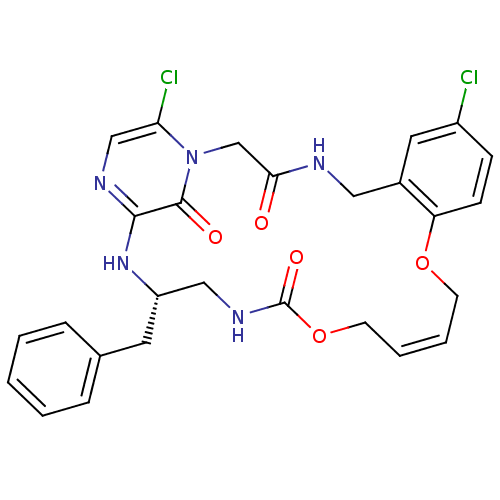
((14E,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C\COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |t:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4+/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tissue plasminogen activator |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14144
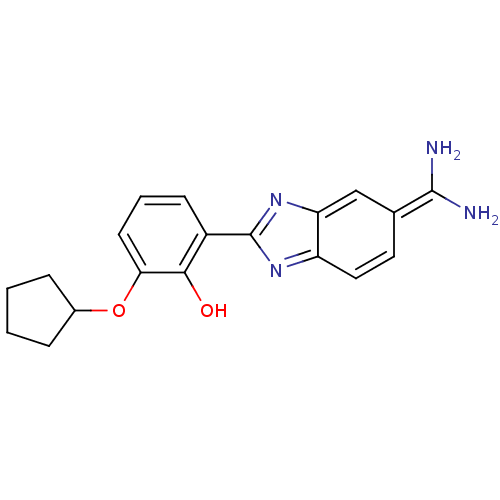
(2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...)Show SMILES NC(=[NH2+])c1ccc2nc([nH]c2c1)-c1cccc(OC2CCCC2)c1[O-] Show InChI InChI=1S/C19H20N4O2/c20-18(21)11-8-9-14-15(10-11)23-19(22-14)13-6-3-7-16(17(13)24)25-12-4-1-2-5-12/h3,6-10,12,24H,1-2,4-5H2,(H3,20,21)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data