

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit tissue-type plasminogen activator (t-PA) was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298092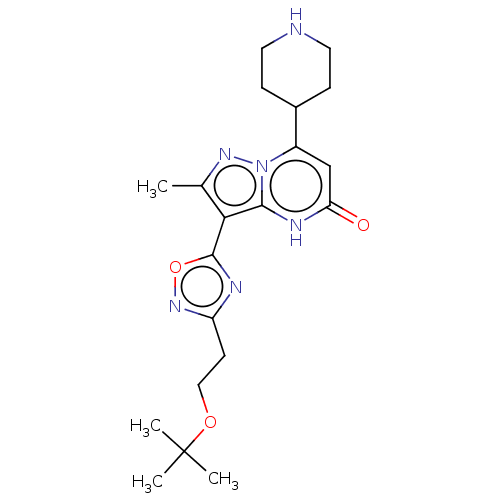 (3-[3-(2-tert-butoxyethyl)-1,2,4-oxadiazol-5-yl]-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298254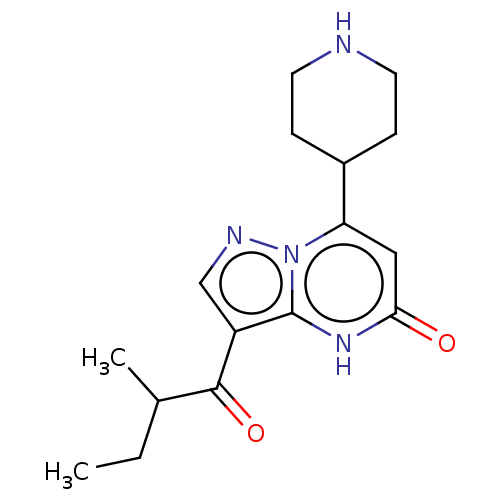 (3-(2-methylbutanoyl)-7-(piperidin-4-yl)pyrazolo[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298255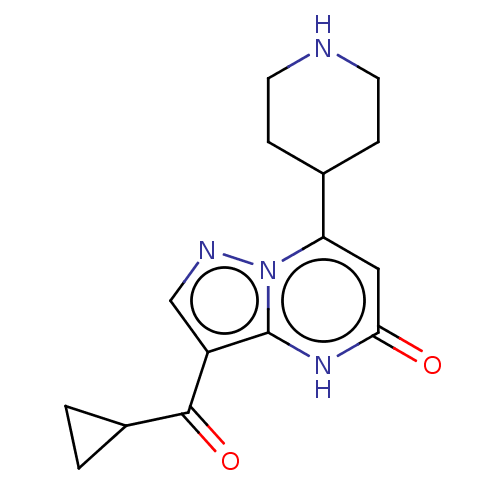 (3-(cyclopropylcarbonyl)-7-(piperidin-4-yl)pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM92479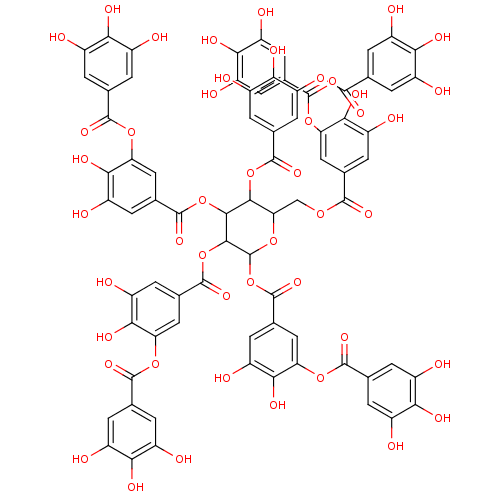 (Tannic Acid, A | Tannic acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298041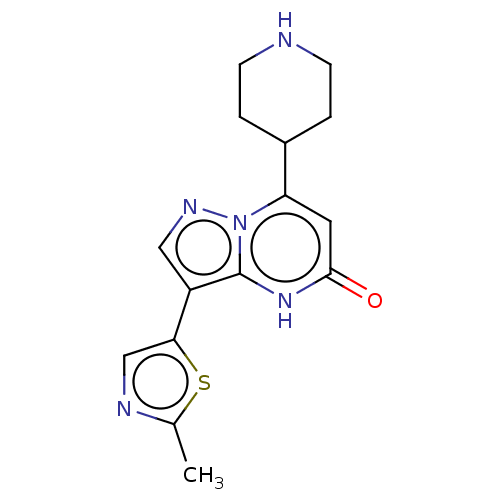 (3-(2-methyl-1,3-thiazol-5-yl)-7-(piperidin-4-yl)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297950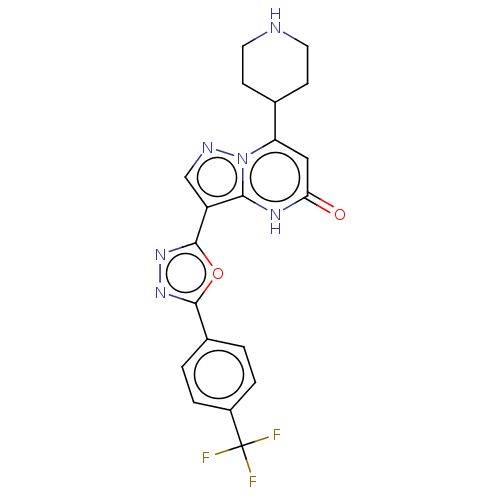 (7-(piperidin-4-yl)-3-{5-[4-(trifluoromethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298115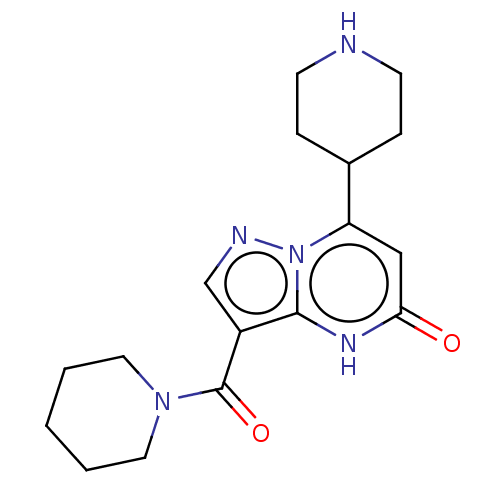 (7-(piperidin-4-yl)-3-(piperidin-1-ylcarbonyl)pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298123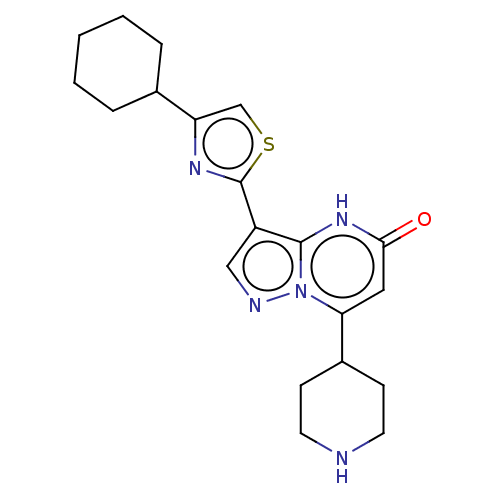 (3-(4-cyclohexyl-1,3-thiazol-2-yl)-7-(piperidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50029498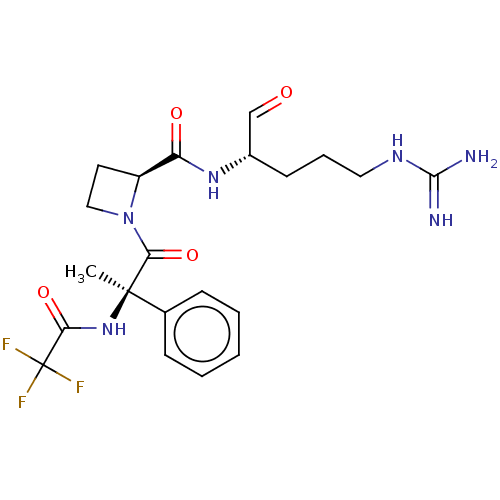 (1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity against t-PA(Tissue plasminogen activator). | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298009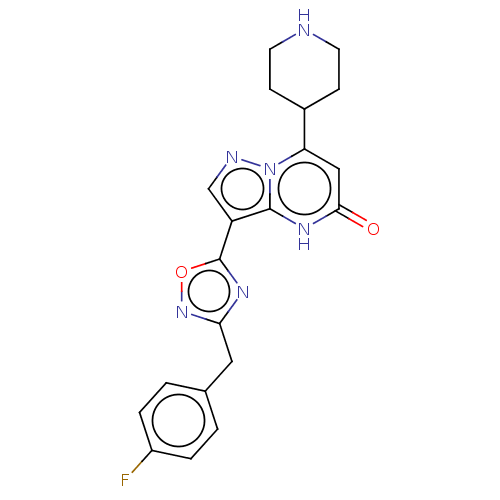 (3-[3-(4-fluorobenzyl)-1,2,4-oxadiazol-5-yl]-7-(pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298237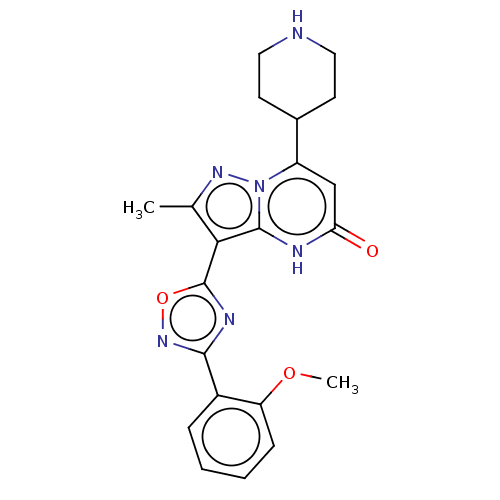 (3-[3-(2-methoxyphenyl)-1,2,4-oxadiazol-5-yl]-2-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298073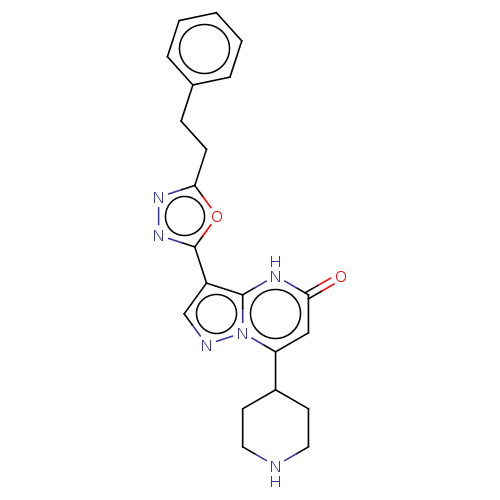 (3-[5-(2-phenylethyl)-1,3,4-oxadiazol-2-yl]-7-(pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298184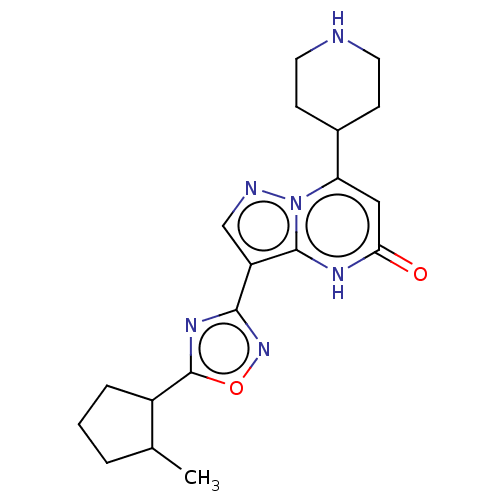 (Rel-3-{5-[(1R,2S)-2-methylcyclopentyl]-1,2,4-oxadi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298246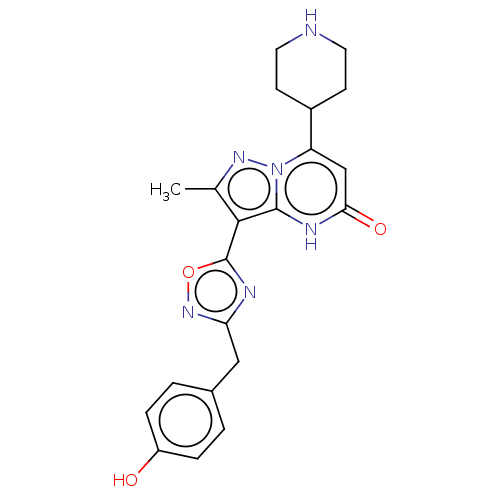 (3-[3-(4-hydroxybenzyl)-1,2,4-oxadiazol-5-yl]-2-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298253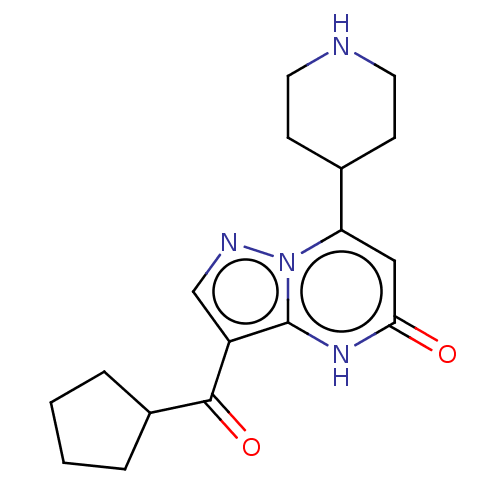 (3-(cyclopentylcarbonyl)-7-(piperidin-4-yl)pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298127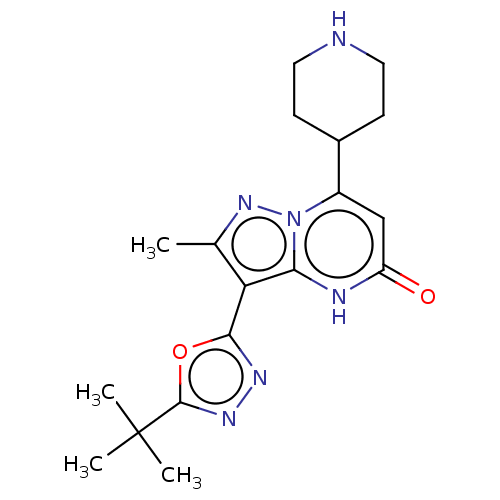 (3-(5-tert-butyl-1,3,4-oxadiazol-2-yl)-2-methyl-7-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298161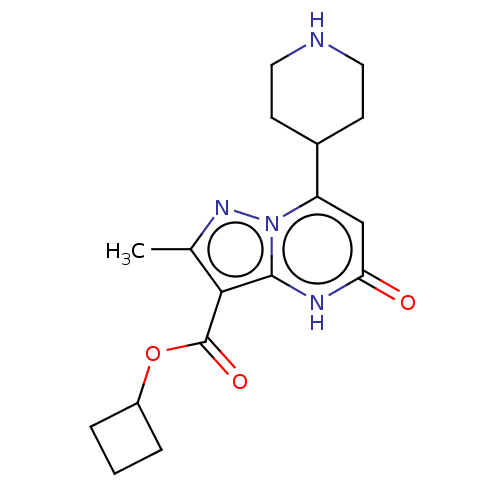 (Cyclobutyl 2-methyl-5-oxo-7-(piperidin-4-yl)-4,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298170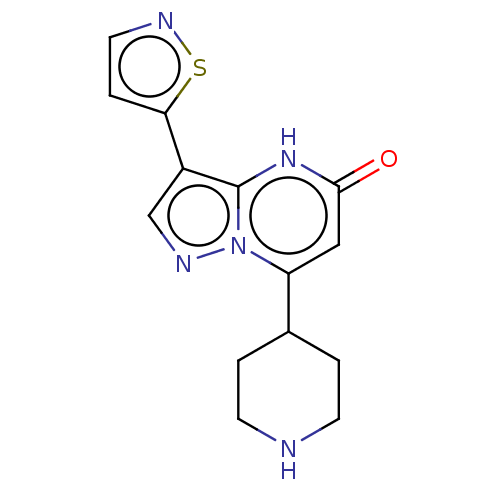 (7-(piperidin-4-yl)-3-(1,2-thiazol-5-yl)pyrazolo[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297845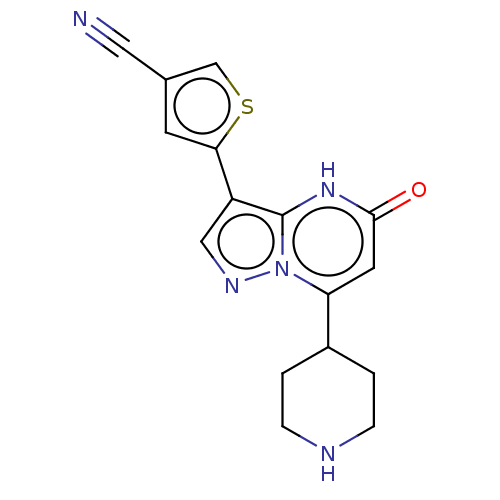 (5-[5-Oxo-7-(piperidin-4-yl)-4,5-dihydropyrazolo[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298096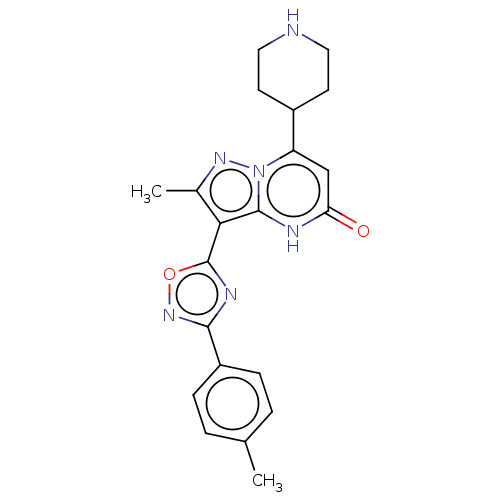 (2-methyl-3-[3-(4-methylphenyl)-1,2,4-oxadiazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298098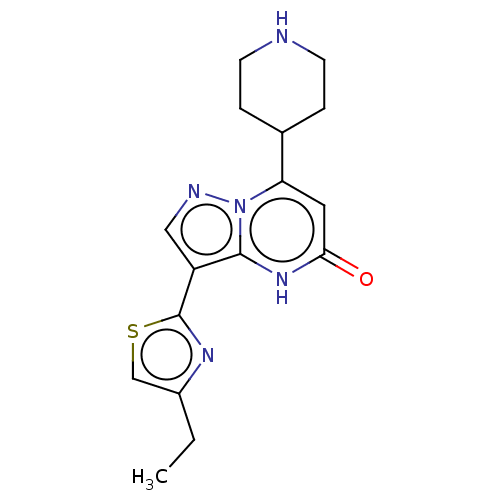 (3-(4-ethyl-1,3-thiazol-2-yl)-7-(piperidin-4-yl)pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297791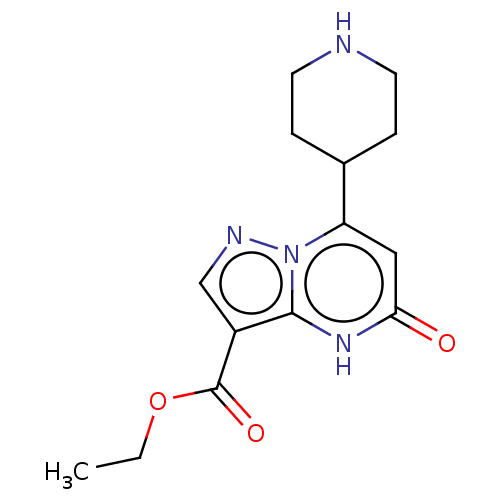 (Ethyl 5-oxo-7-(piperidin-4-yl)-4,5-dihydropyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298063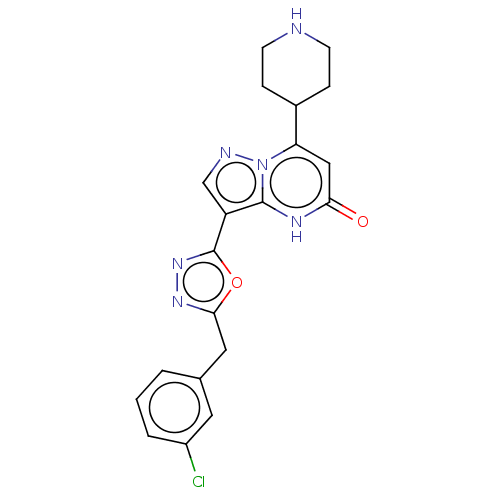 (3-[5-(3-chlorobenzyl)-1,3,4-oxadiazol-2-yl]-7-(pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298238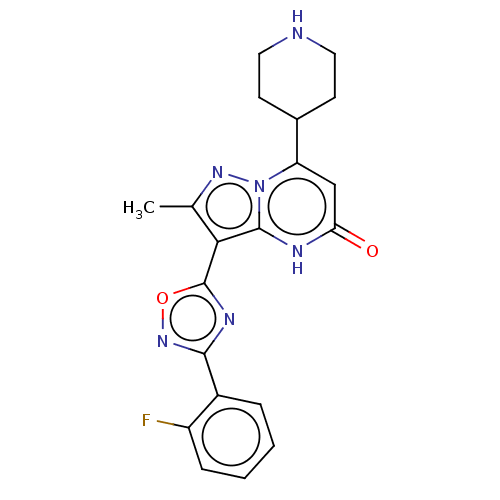 (3-[3-(2-fluorophenyl)-1,2,4-oxadiazol-5-yl]-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298114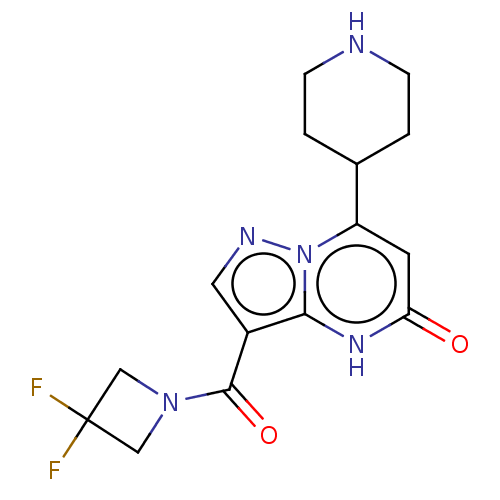 (3-[(3,3-difluoroazetidin-1-yl)carbonyl]-7-(piperid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298037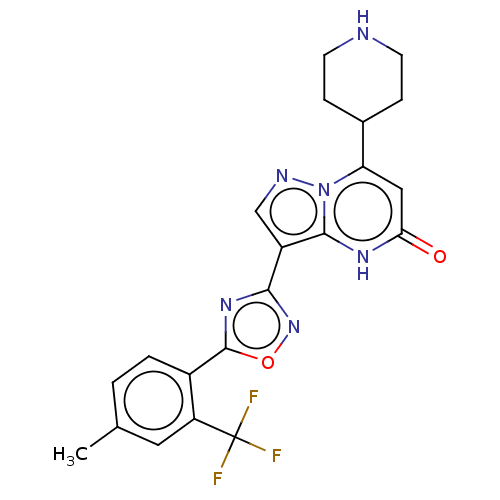 (3-{5-[4-methyl-2-(trifluoromethyl)phenyl]-1,2,4-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297842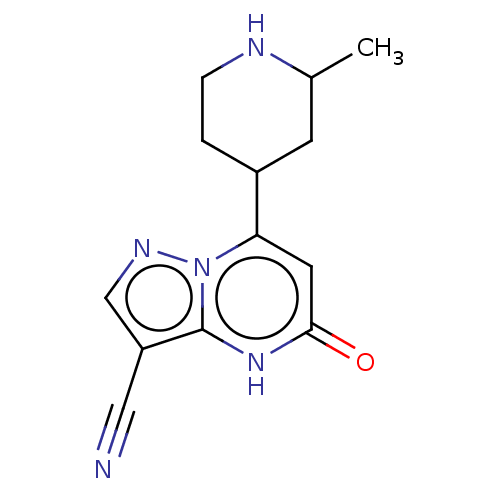 ((−)-trans-7-(2-Methylpiperidin-4-yl)-5-oxo-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM92485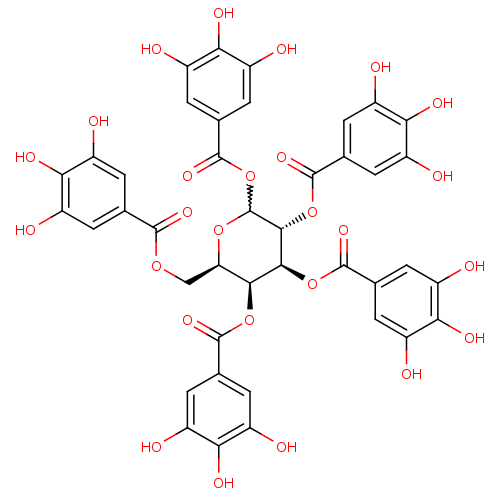 (CDE-066 | US9120744, CDE-066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297809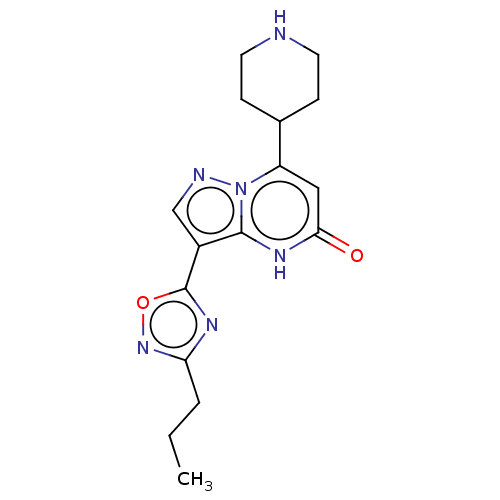 (7-(Piperidin-4-yl)-3-(3-propyl-1,2,4-oxadiazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297850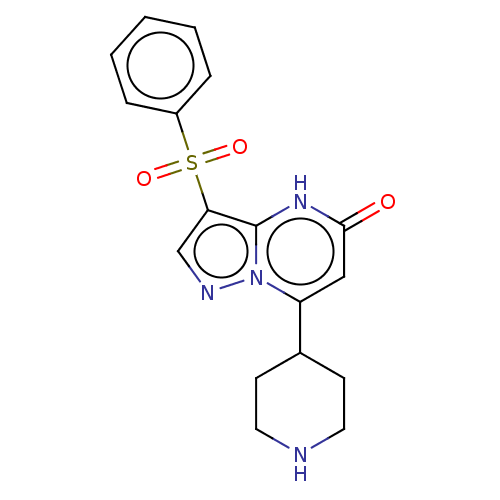 (3-(Phenylsulfonyl)-7-(piperidin-4-yl)pyrazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298061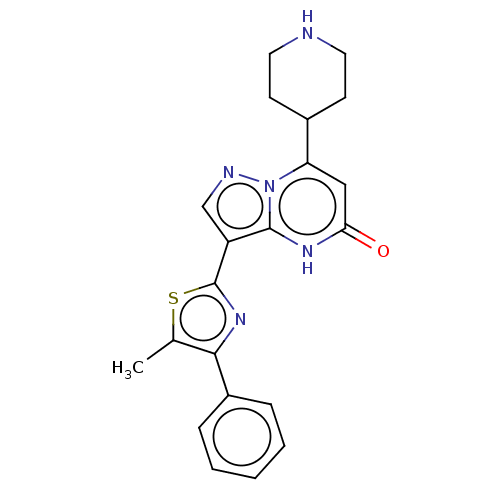 (3-(5-methyl-4-phenyl-1,3-thiazol-2-yl)-7-(piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297797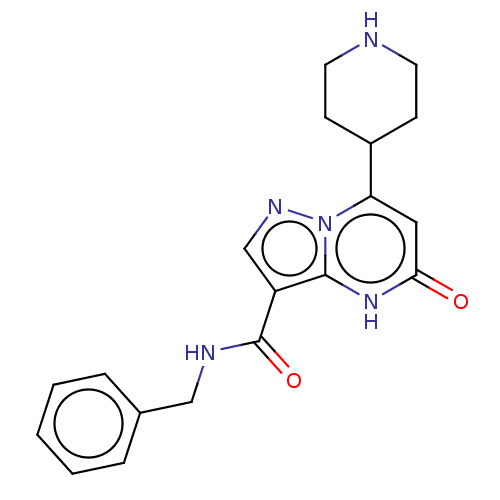 (N-Benzyl-5-oxo-7-(piperidin-4-yl)-4,5-dihydropyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297837 (3-(3-Methoxyphenyl)-7-(piperidin-4-yl)pyrazolo[1,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297824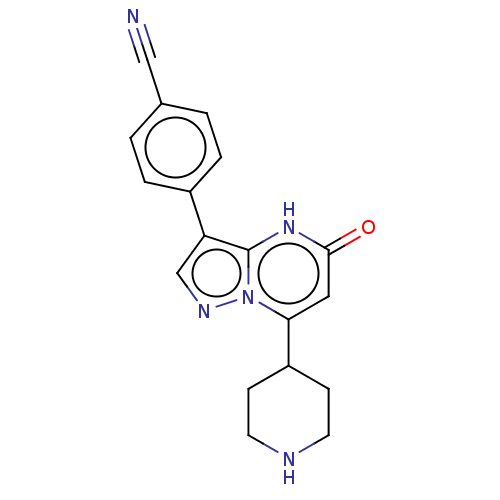 (4-[5-Oxo-7-(piperidin-4-yl)-4,5-dihydropyrazolo[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297808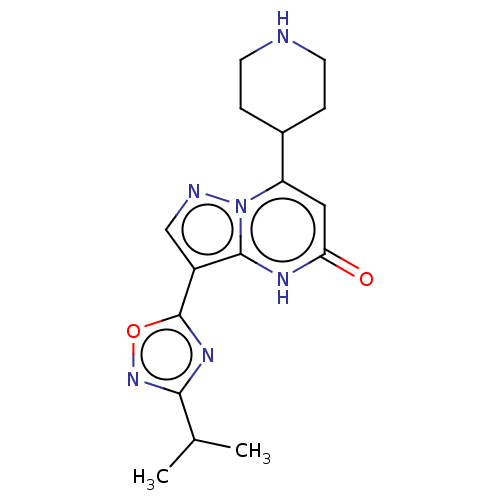 (3-(3-Isopropyl-1,2,4-oxadiazol-5-yl)-7-(piperidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298247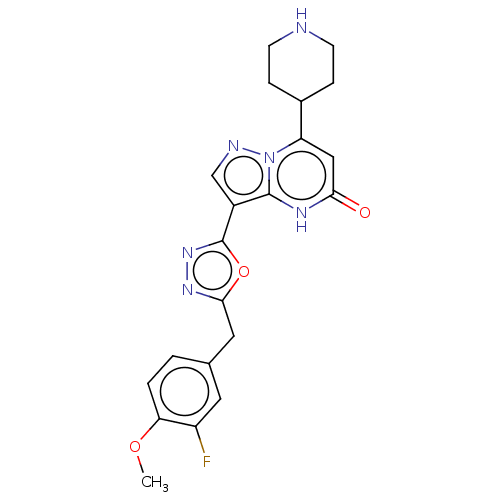 (3-[5-(3-fluoro-4-methoxybenzyl)-1,3,4-oxadiazol-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298154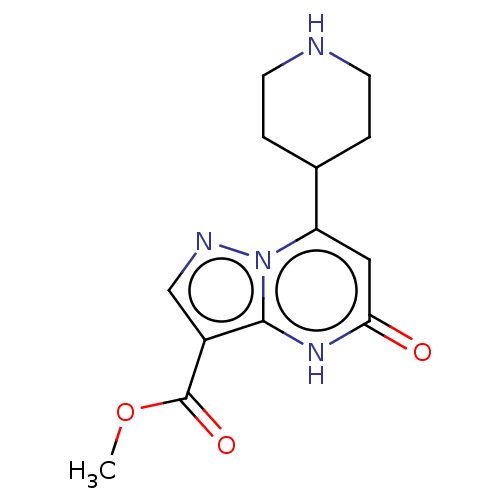 (Methyl 5-oxo-7-(piperidin-4-yl)-4,5-dihydropyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298177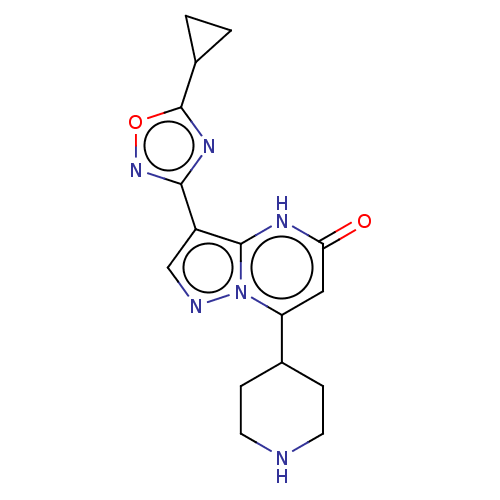 (3-(5-Cyclopropyl-1,2,4-oxadiazol-3-yl)-7-(piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298182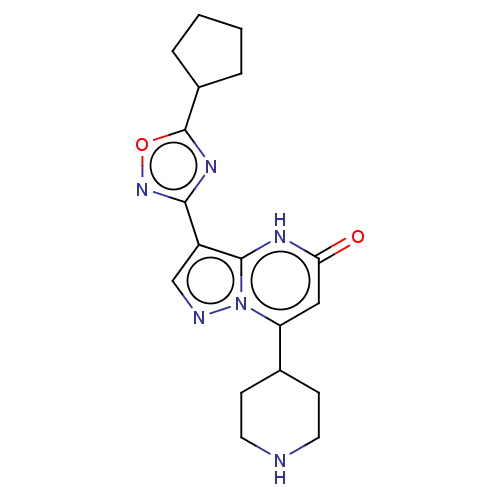 (3-(5-Cyclopentyl-1,2,4-oxadiazol-3-yl)-7-(piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298065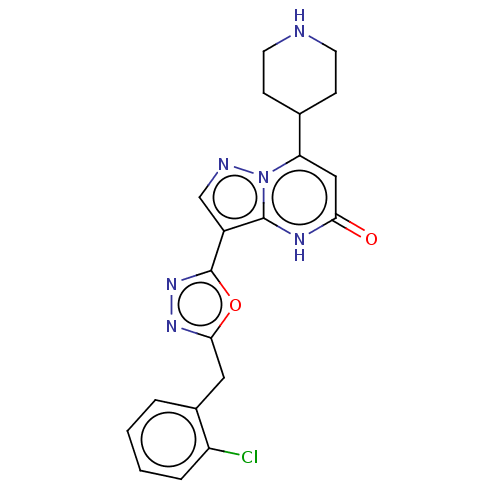 (3-[5-(2-chlorobenzyl)-1,3,4-oxadiazol-2-yl]-7-(pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297825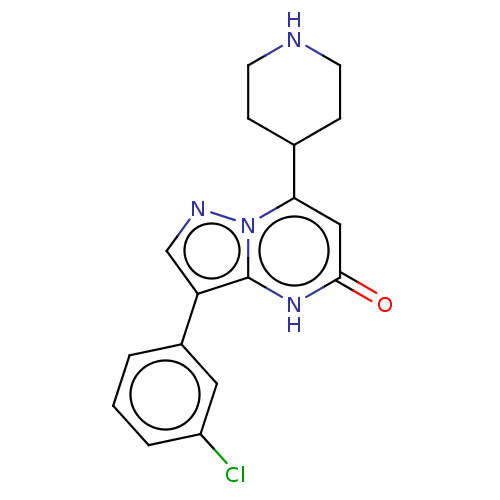 (3-(3-Chlorophenyl)-7-(piperidin-4-yl)pyrazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297939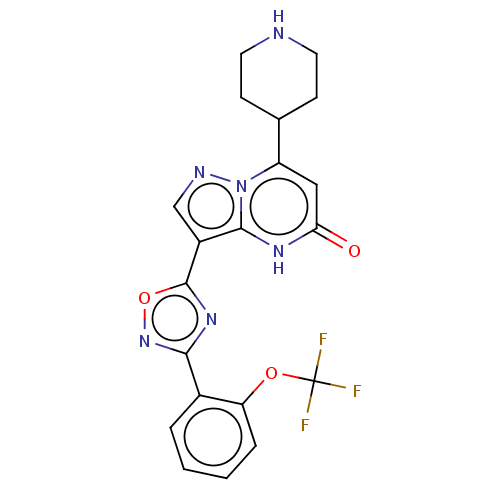 (7-(piperidin-4-yl)-3-{3-[2-(trifluoromethoxy)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297796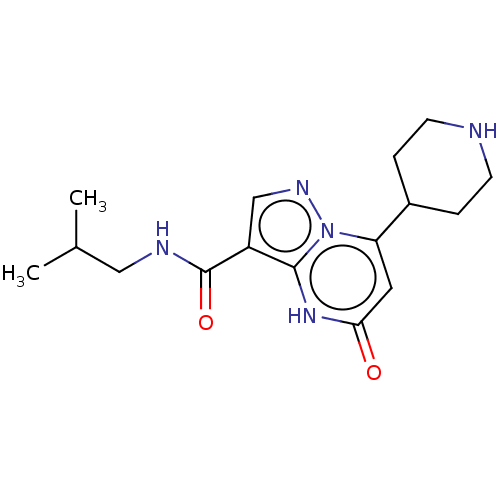 (N-Isobutyl-5-oxo-7-(piperidin-4-yl)-4,5-dihydropyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297798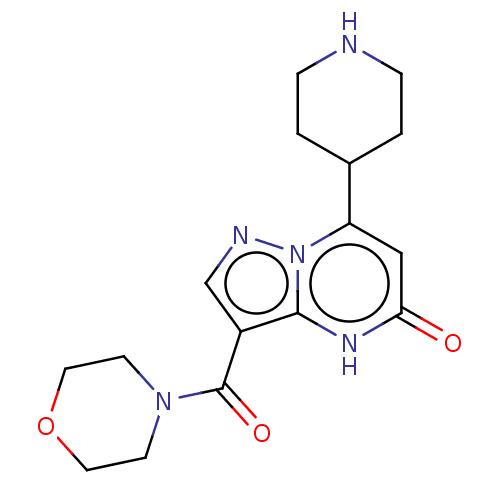 (3-(Morpholin-4-ylcarbonyl)-7-(piperidin-4-yl)pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297815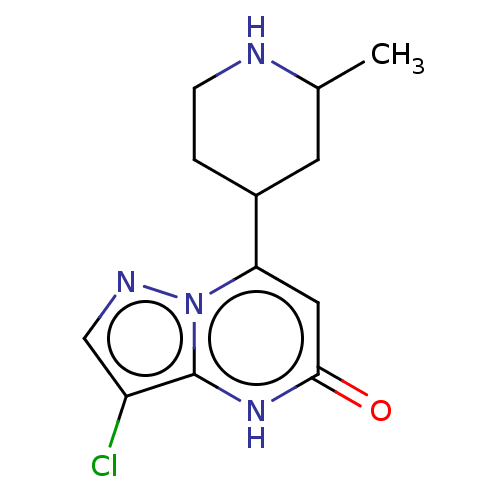 ((−)-trans-3-Chloro-7-(2-methylpiperidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM297847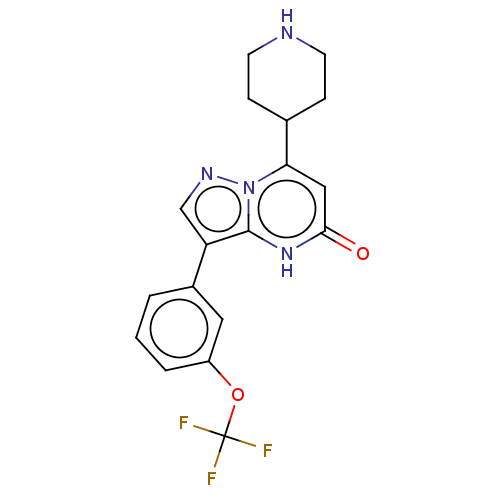 (7-(Piperidin-4-yl)-3-[3-(trifluoromethoxy)phenyl]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298180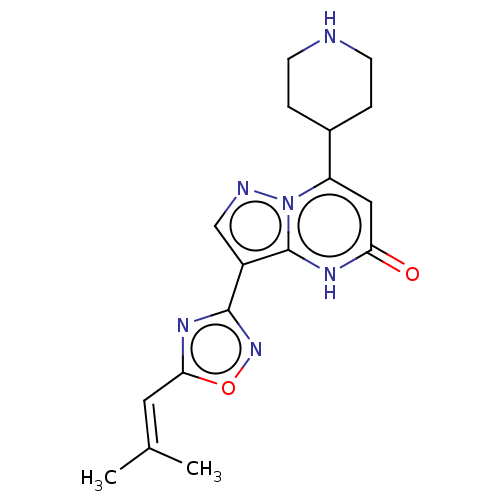 (3-[5-(2-Methylprop-1-en-1-yl)-1,2,4-oxadiazol-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298055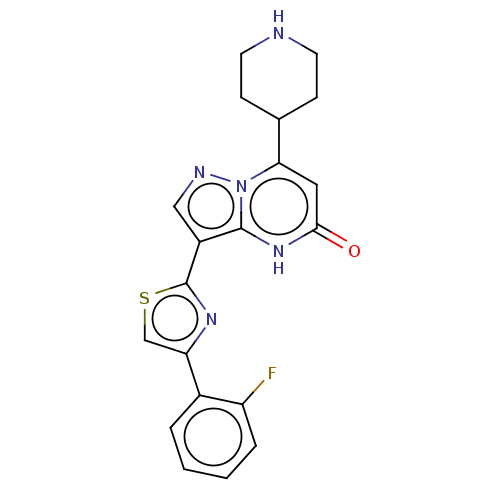 (3-[4-(2-fluorophenyl)-1,3-thiazol-2-yl]-7-(piperid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM298005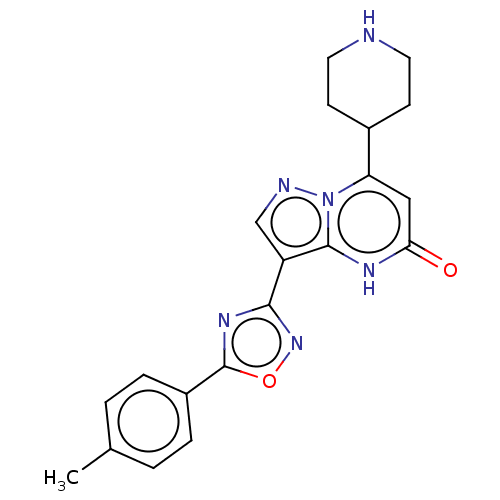 (3-[5-(4-methylphenyl)-1,2,4-oxadiazol-3-yl]-7-(pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel... | US Patent US10118930 (2018) BindingDB Entry DOI: 10.7270/Q2QC05J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 769 total ) | Next | Last >> |