

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GTPase HRas (Homo sapiens (Human)) | BDBM50092365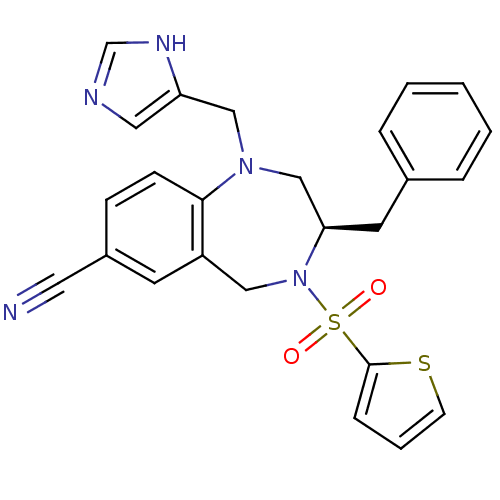 ((R)-1-((1H-imidazol-5-yl)methyl)-3-benzyl-4-(thiop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113877 BindingDB Entry DOI: 10.7270/Q2PK0M78 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50092365 ((R)-1-((1H-imidazol-5-yl)methyl)-3-benzyl-4-(thiop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113006 BindingDB Entry DOI: 10.7270/Q2G44VBP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089967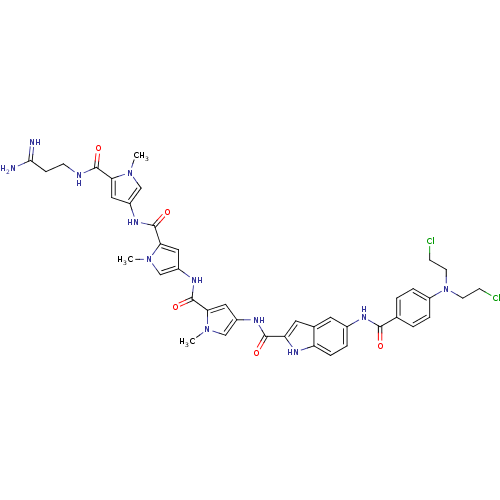 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089960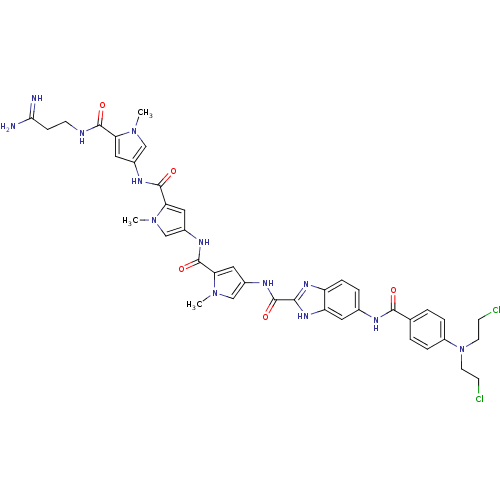 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089962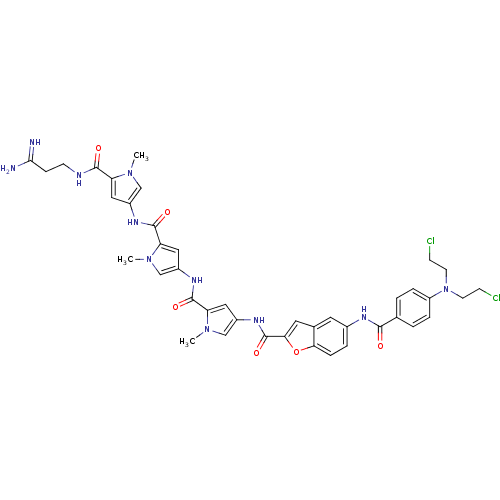 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089961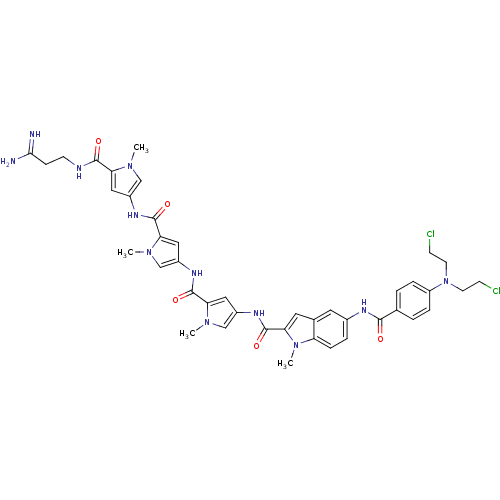 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089969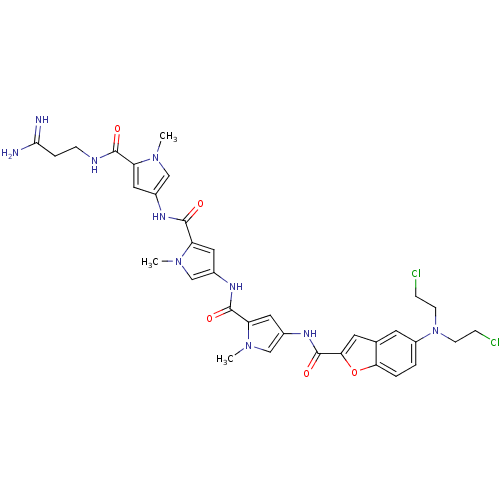 (5-[Bis-(2-chloro-ethyl)-amino]-benzofuran-2-carbox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089971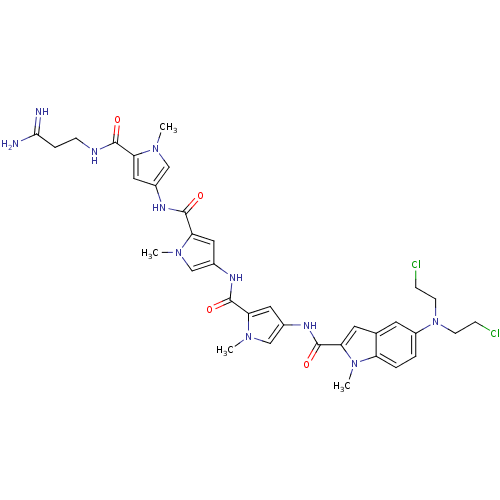 (5-[Bis-(2-chloro-ethyl)-amino]-1-methyl-1H-indole-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089978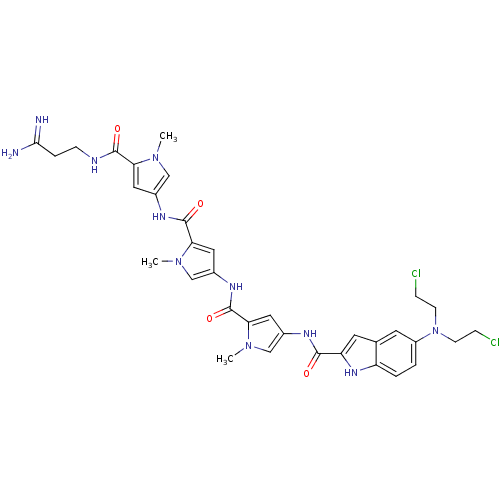 (5-[Bis-(2-chloro-ethyl)-amino]-1H-indole-2-carboxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060139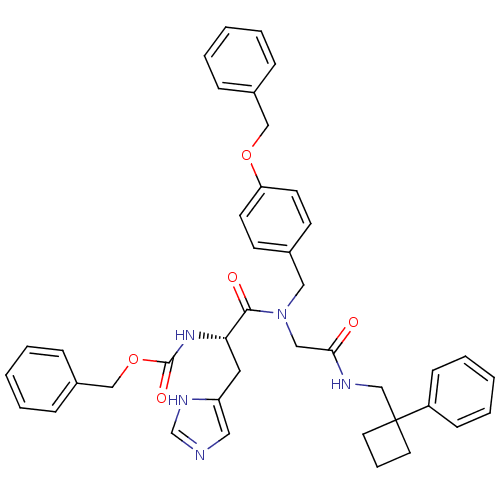 (CHEMBL326352 | [(S)-1-((4-Benzyloxy-benzyl)-{[(1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50601398 (CHEMBL5174384) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113006 BindingDB Entry DOI: 10.7270/Q2G44VBP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50601400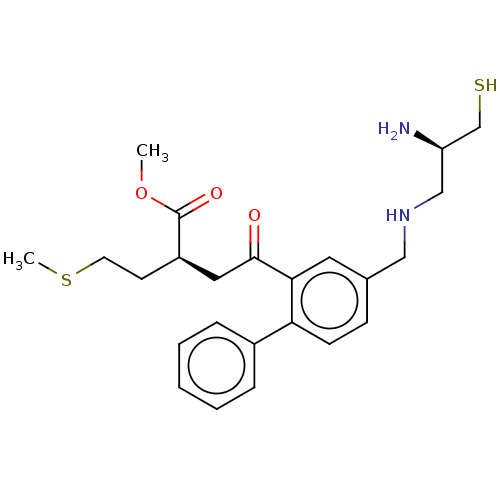 (CHEMBL5171806) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113006 BindingDB Entry DOI: 10.7270/Q2G44VBP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060141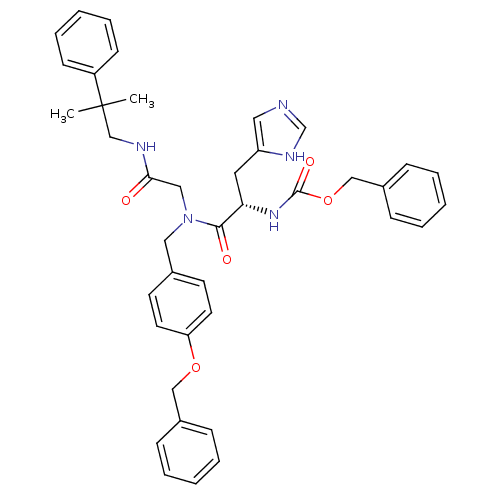 (CHEMBL431440 | [(S)-1-{(4-Benzyloxy-benzyl)-[(2-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060146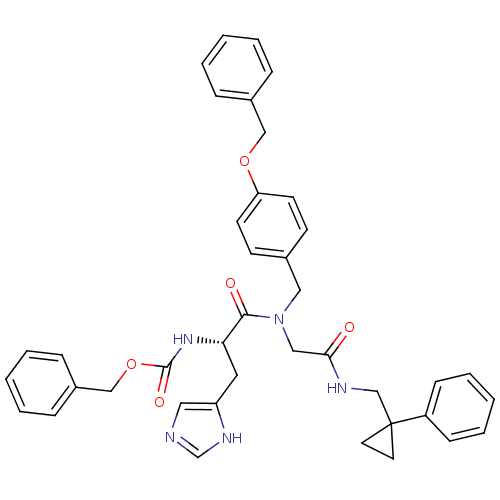 (CHEMBL407445 | [(S)-1-((4-Benzyloxy-benzyl)-{[(1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060140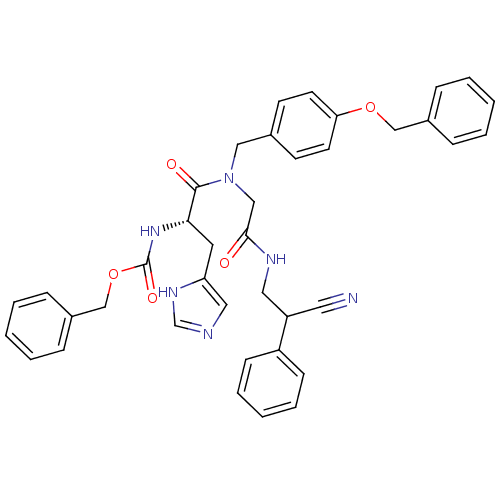 (CHEMBL115943 | [(S)-1-{(4-Benzyloxy-benzyl)-[(2-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50055659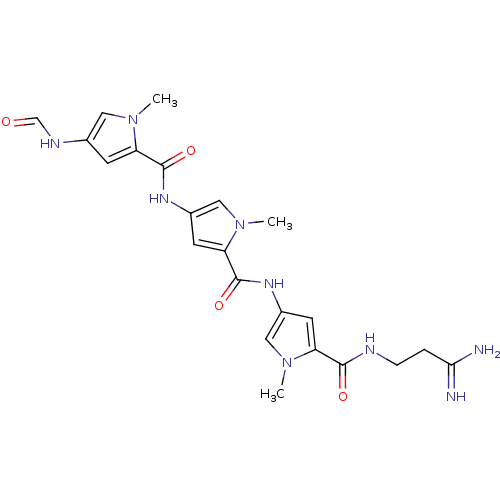 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060145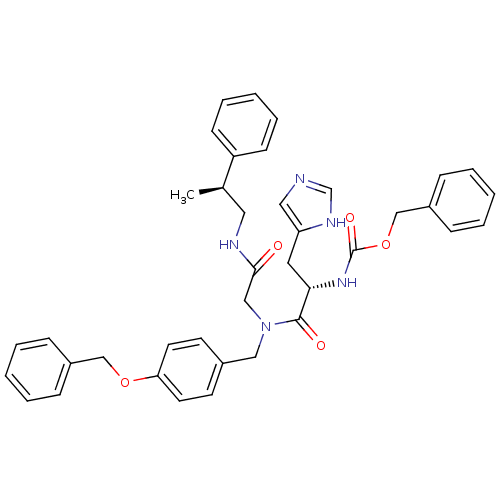 (CHEMBL115669 | [(S)-1-{(4-Benzyloxy-benzyl)-[((S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060138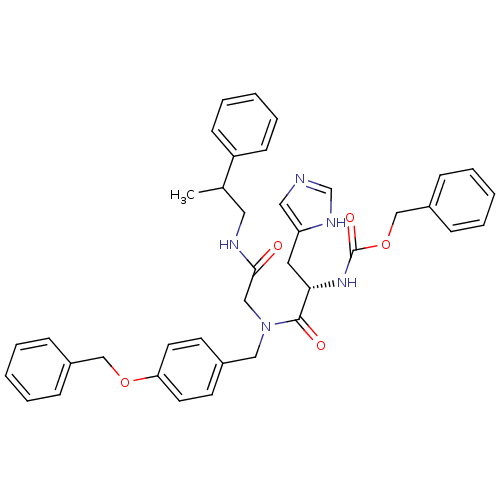 (CHEMBL324827 | [(S)-1-{(4-Benzyloxy-benzyl)-[(2-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060142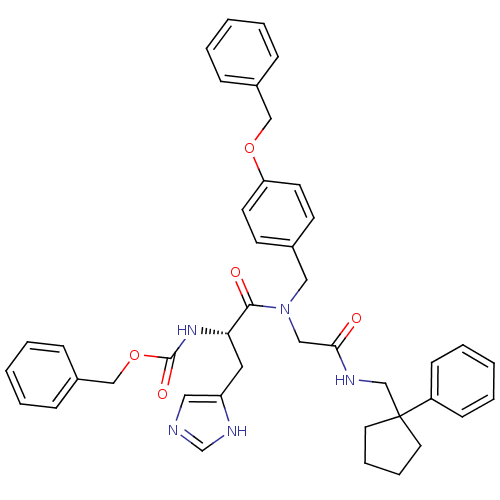 (CHEMBL326127 | [(S)-1-((4-Benzyloxy-benzyl)-{[(1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060149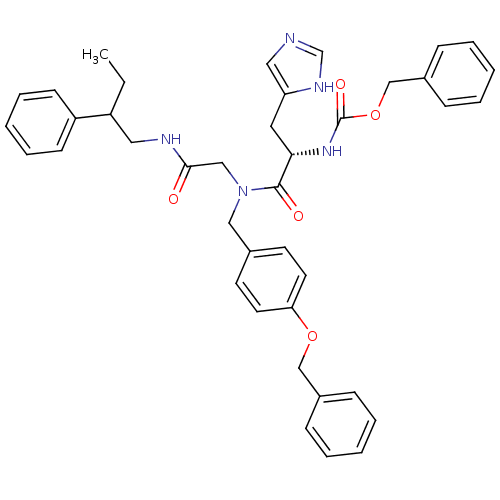 (CHEMBL112512 | [(S)-1-{(4-Benzyloxy-benzyl)-[(2-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060150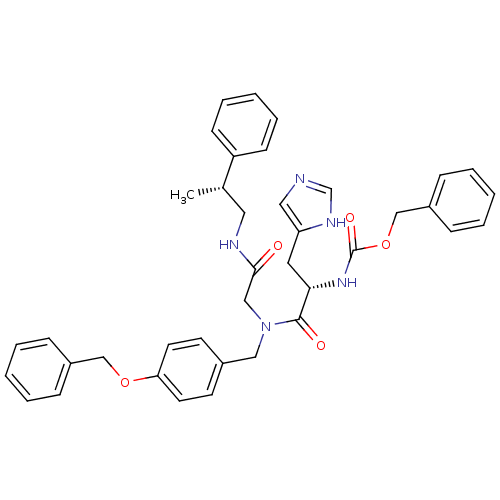 (CHEMBL114281 | [(S)-1-{(4-Benzyloxy-benzyl)-[((R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060144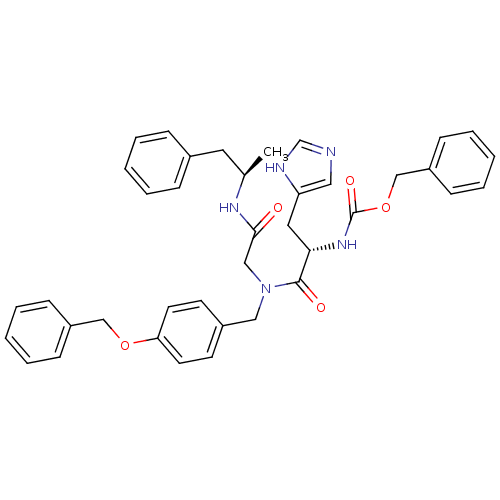 (CHEMBL325073 | [(S)-1-{(4-Benzyloxy-benzyl)-[((R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060148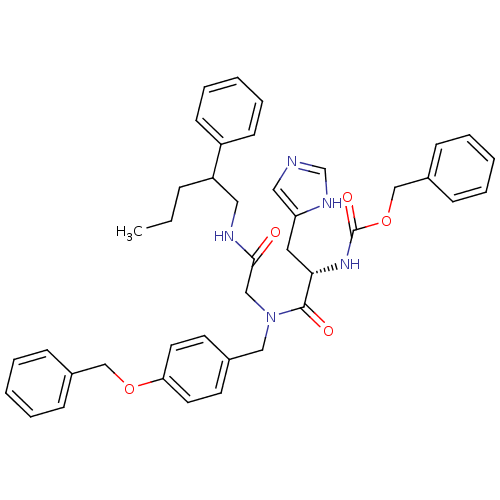 (CHEMBL325331 | [(S)-1-{(4-Benzyloxy-benzyl)-[(2-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060152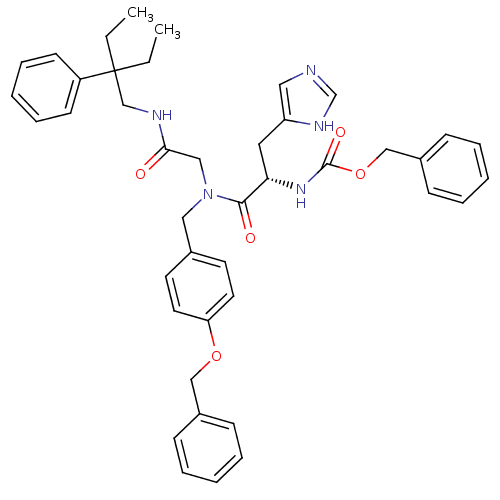 (CHEMBL112879 | [(S)-1-{(4-Benzyloxy-benzyl)-[(2-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287960 (1-[(2R,6S)-6-(tert-Butyl-dimethyl-silanyloxymethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287951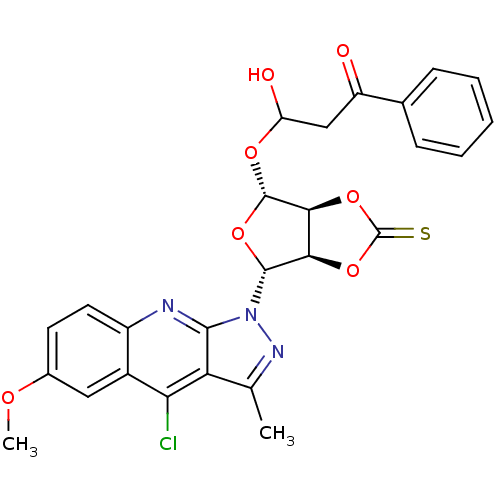 (3-[(3aS,4R,6R,6aR)-6-(4-Chloro-6-methoxy-3-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50478506 (Ophirapstanol Trisulfate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harbor Branch Oceanographic Institution, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant p21RAS by GDPX assay | J Nat Prod 57: 1751-4 (1994) Article DOI: 10.1021/np50114a024 BindingDB Entry DOI: 10.7270/Q261133S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287961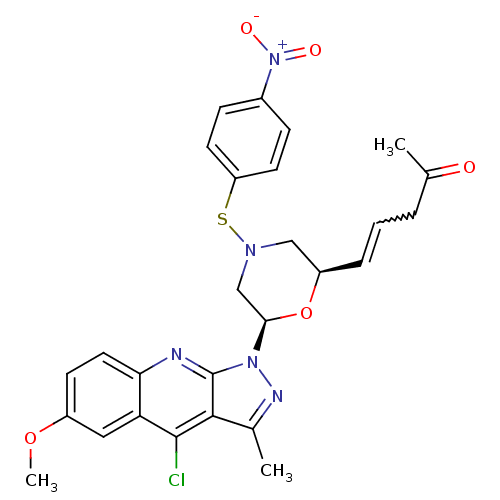 ((E)-5-[(2R,6R)-6-(4-Chloro-6-methoxy-3-methyl-pyra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50059857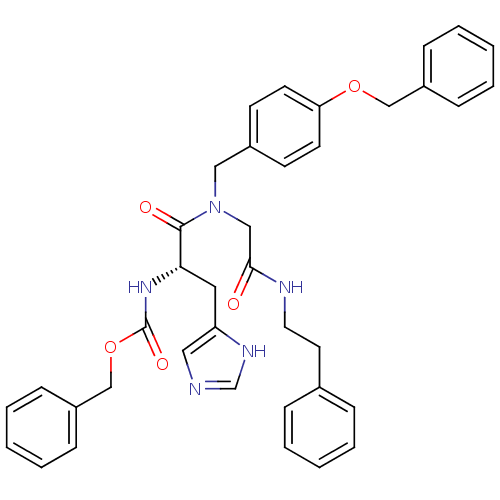 (CHEMBL102955 | [(S)-1-[(4-Benzyloxy-benzyl)-(phene...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287943 ((E)-4-[(2R,6R)-6-(4-Chloro-6-methoxy-3-methyl-pyra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287946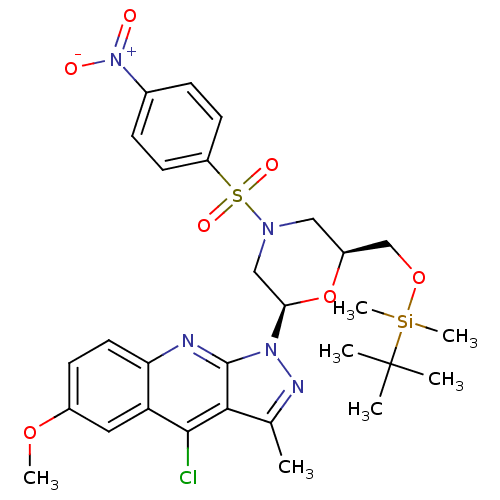 (1-[(2R,6S)-6-(tert-Butyl-dimethyl-silanyloxymethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50060143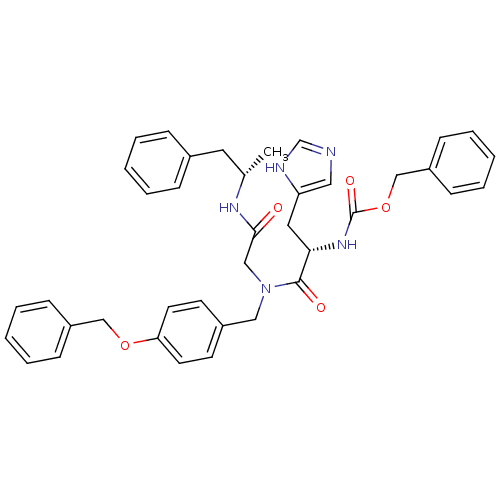 (CHEMBL324345 | [(S)-1-{(4-Benzyloxy-benzyl)-[((S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287963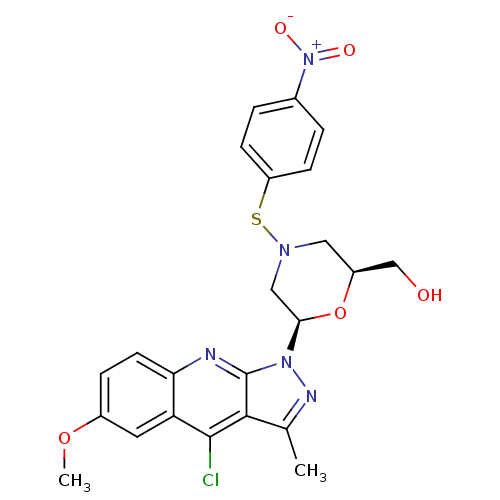 (CHEMBL300769 | [(2S,6R)-6-(4-Chloro-6-methoxy-3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287959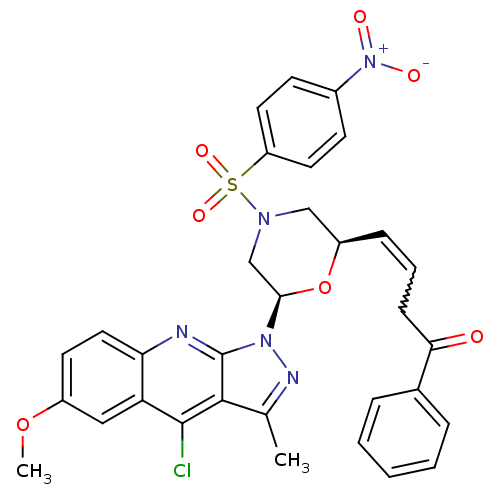 ((E)-4-[(2R,6R)-6-(4-Chloro-6-methoxy-3-methyl-pyra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287955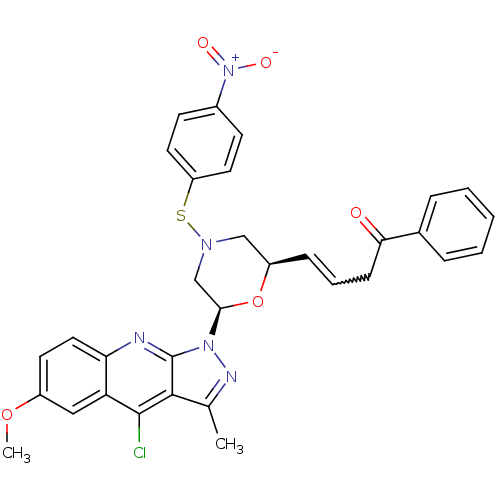 ((E)-4-[(2R,6R)-6-(4-Chloro-6-methoxy-3-methyl-pyra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287958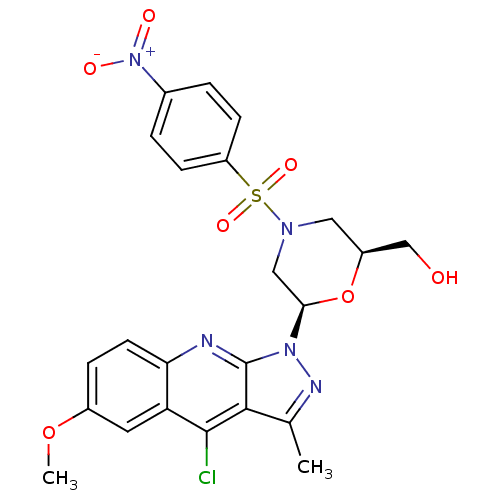 (CHEMBL303105 | [(2S,6R)-6-(4-Chloro-6-methoxy-3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50072339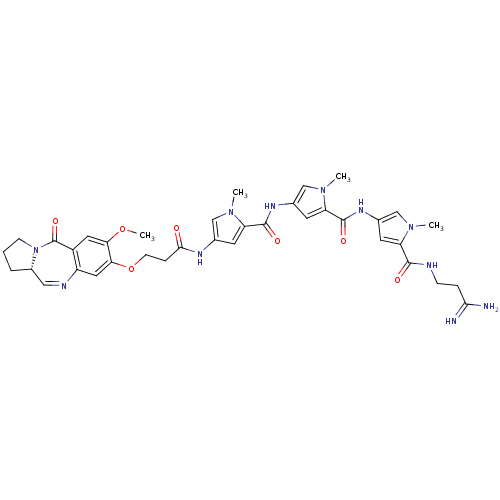 (Benzodiazepine-Distamycin Hybrid | CHEMBL102341) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of Ha-ras polymerase-chain reaction product | Bioorg Med Chem Lett 8: 3019-24 (1999) Article DOI: 10.1016/S0960-894X(98)00544-7 BindingDB Entry DOI: 10.7270/Q2057GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287944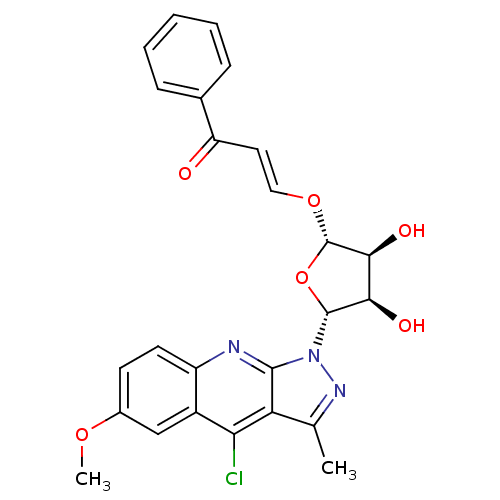 ((E)-3-[(2S,3S,4R,5R)-5-(4-Chloro-6-methoxy-3-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287945 (5-(4-chloro-6-methoxy-3-methyl-1H-pyrazolo[3,4-b]q...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro for inhibition of nucleotide exchange process of oncogenic Ras | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287953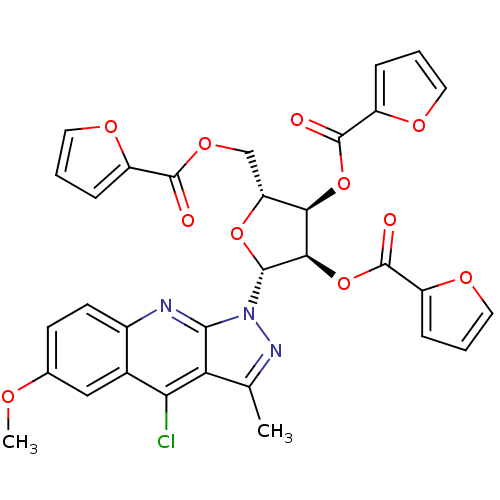 (5-(4-chloro-6-methoxy-3-methyl-1H-pyrazolo[3,4-b]q...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287962 (CHEMBL61870 | [(2S,6R)-4-Benzenesulfonyl-6-(4-chlo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287949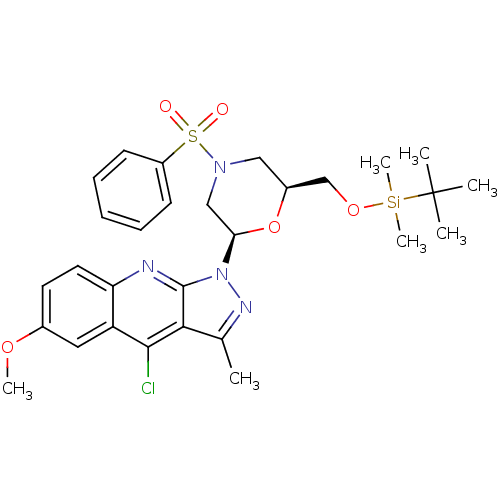 (1-[(2R,6S)-4-Benzenesulfonyl-6-(tert-butyl-dimethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287948 (CHEMBL64886 | [(2S,6R)-6-(4-Chloro-6-methoxy-3-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50059879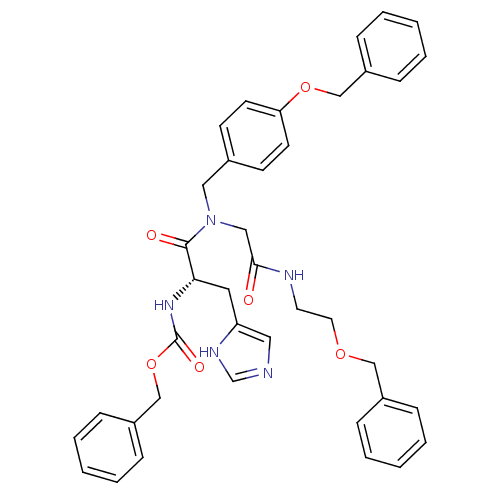 (CHEMBL102492 | [(S)-1-{(4-Benzyloxy-benzyl)-[(2-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of the cultured colonies of H-Ras-Fcells . | J Med Chem 40: 3319-22 (1997) Article DOI: 10.1021/jm970470c BindingDB Entry DOI: 10.7270/Q2V69HQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287956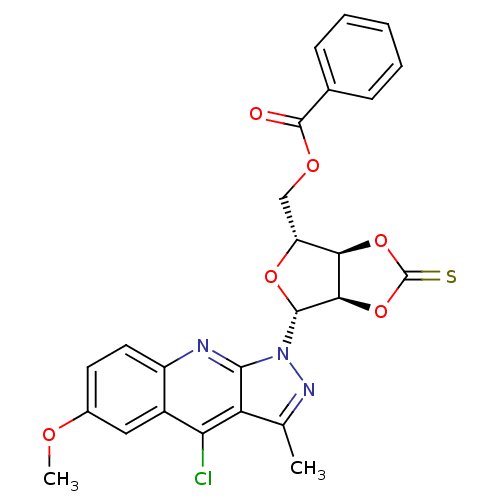 (Benzoic acid (3aR,4R,6R,6aR)-6-(4-chloro-6-methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287947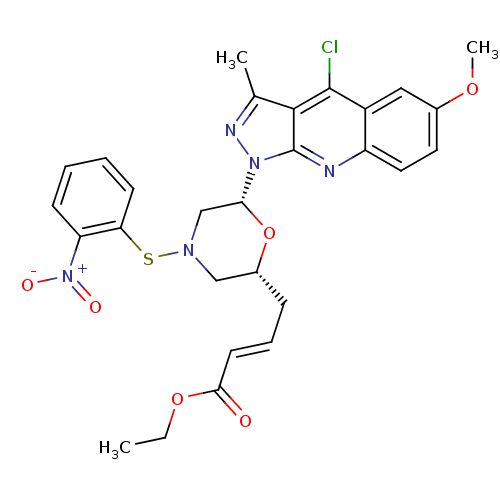 ((E)-4-[(2R,6R)-6-(4-Chloro-6-methoxy-3-methyl-pyra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287952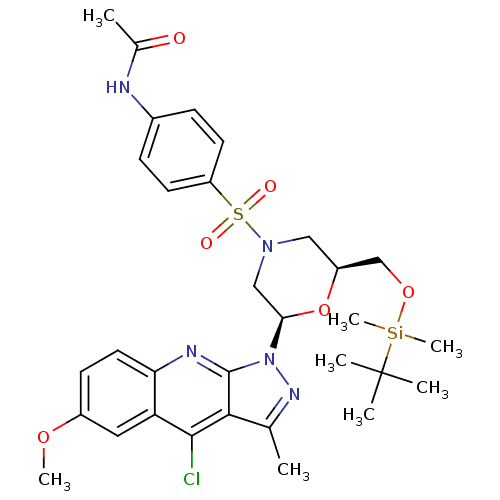 (CHEMBL304717 | N-{4-[(2S,6R)-2-(tert-Butyl-dimethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287954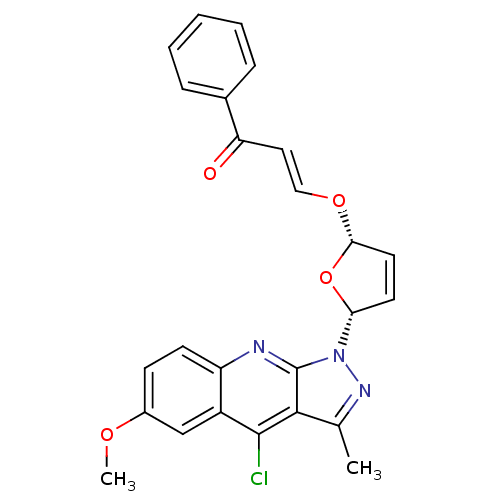 ((E)-3-[(2S,5R)-5-(4-Chloro-6-methoxy-3-methyl-pyra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50601396 (CHEMBL5197181) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113006 BindingDB Entry DOI: 10.7270/Q2G44VBP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50287957 (1-[(2R,6S)-6-(tert-Butyl-dimethyl-silanyloxymethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of nucleotide exchange process of oncogenic Ras in vitro | Bioorg Med Chem Lett 6: 195-200 (1996) Article DOI: 10.1016/0960-894X(95)00574-D BindingDB Entry DOI: 10.7270/Q2G160TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |