

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14969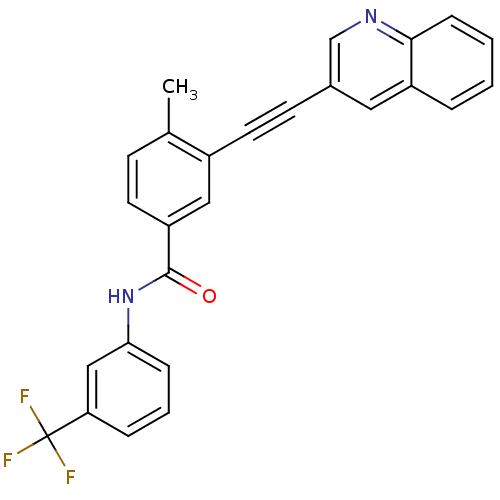 (4-Methyl-3-(2-(quinolin-3-yl)ethynyl)-N-(3-(triflu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14967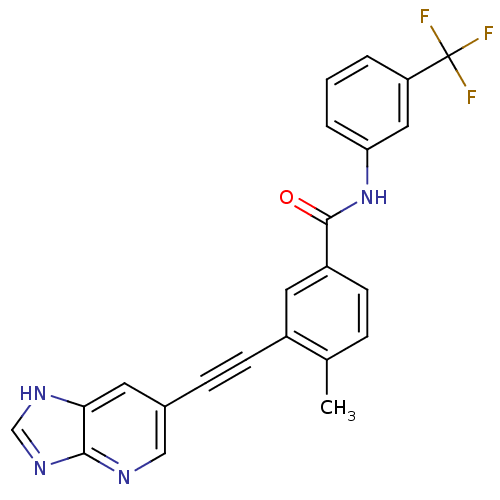 (3-(2-(3H-Imidazo[4,5-b]pyridin-6-yl)ethynyl)-4-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14948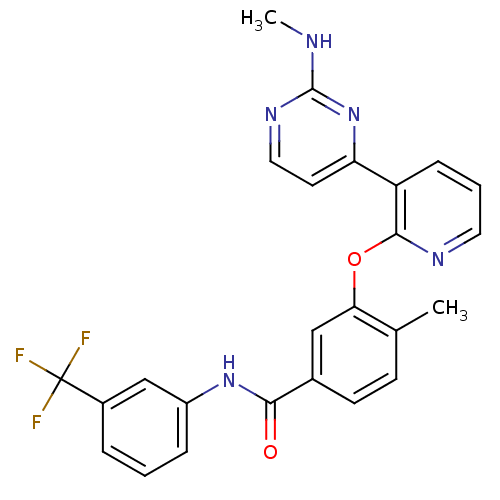 (4-Methyl-3-(3-(2-(methylamino)pyrimidin-4-yl)pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14949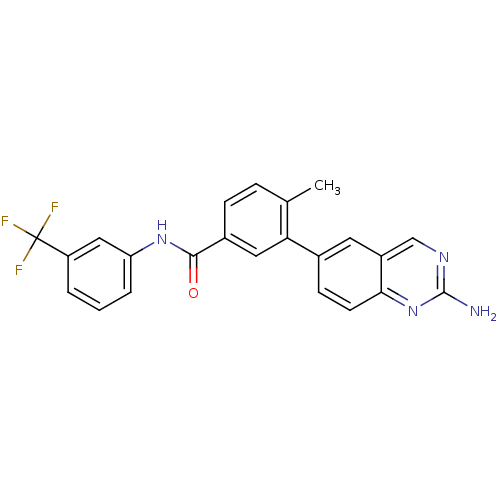 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14950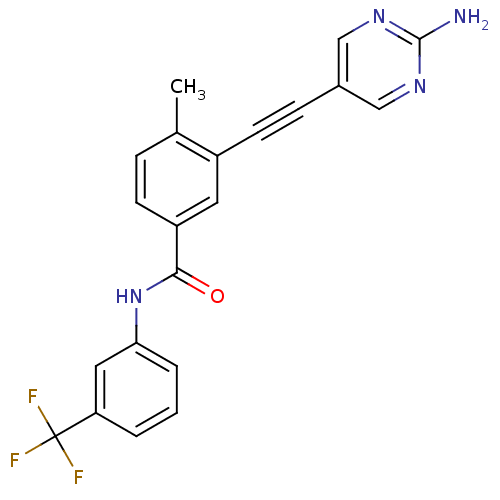 (3-(2-(2-Aminopyrimidin-5-yl)ethynyl)-4-methyl-N-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14966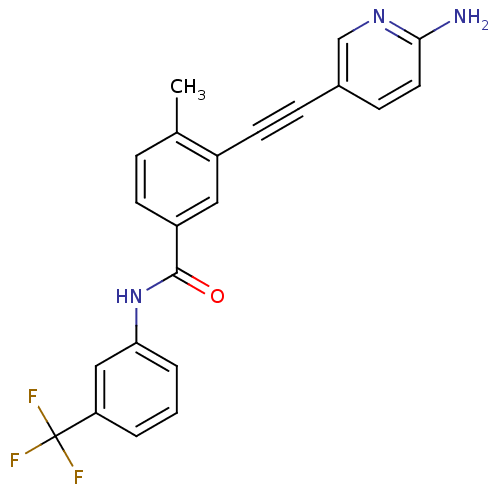 (3-(2-(6-Aminopyridin-3-yl)ethynyl)-4-methyl-N-(3-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14970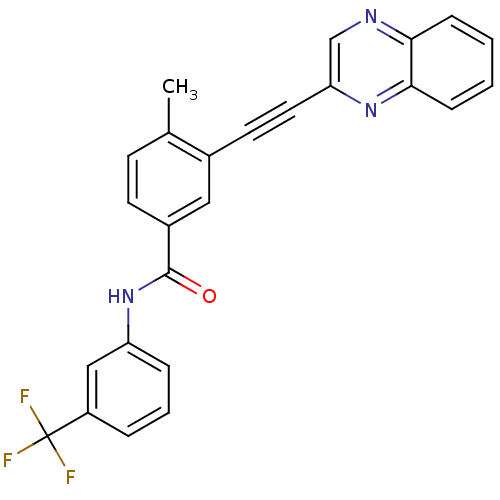 (4-Methyl-3-(2-(quinoxalin-2-yl)ethynyl)-N-(3-(trif...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26166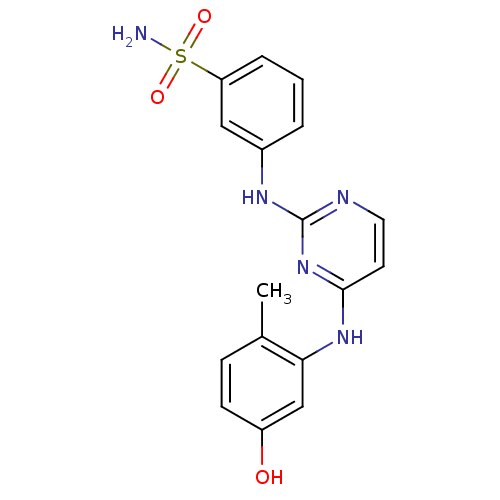 (2,4-dianilino pyrimidine, 23 | 3-({4-[(5-hydroxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26180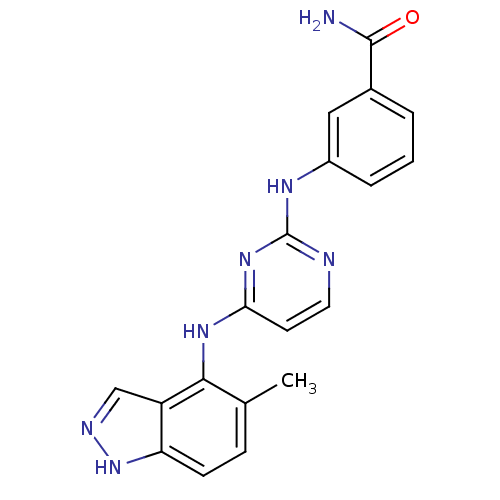 (2,4-dianilino pyrimidine, 37 | 3-({4-[(5-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26145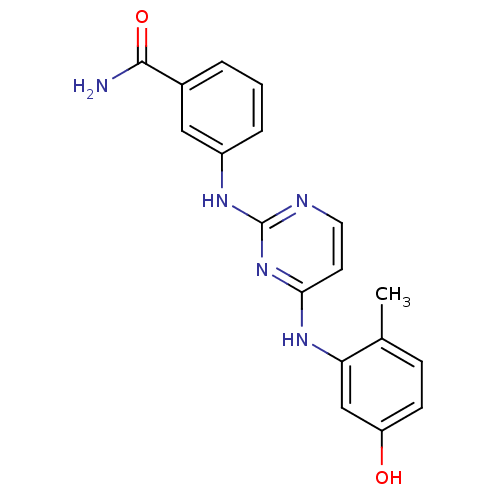 (2,4-dianilino pyrimidine, 2 | 3-({4-[(5-hydroxy-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14951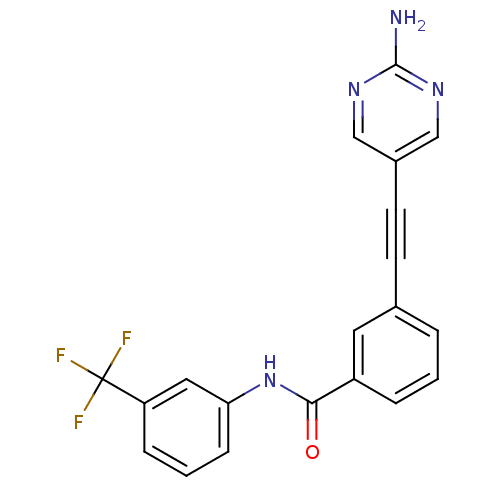 (3-(2-(2-Aminopyrimidin-5-yl)ethynyl)-N-(3-(trifluo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26186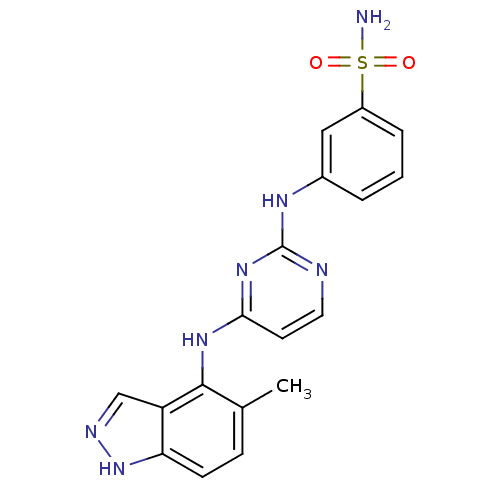 (2,4-dianilino pyrimidine, 43 | 3-({4-[(5-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14957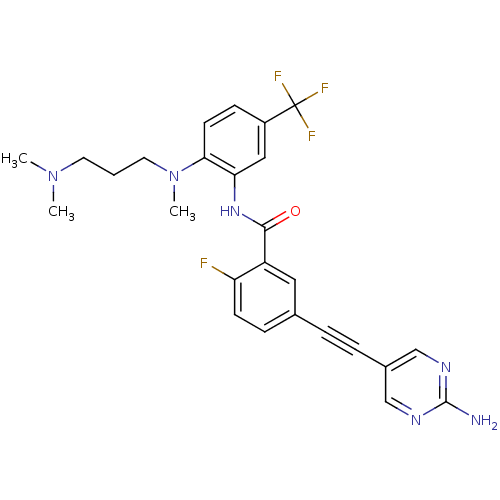 (5-(2-(2-Aminopyrimidin-5-yl)ethynyl)-N-(2-((3-(dim...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50142887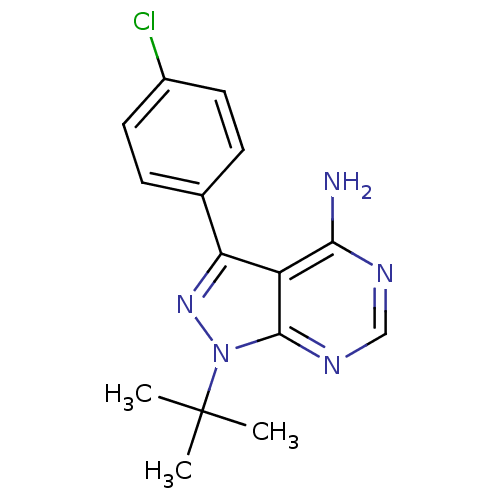 (1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of Lck in the presence of 50uM ATP | Biochem J 408: 297-315 (2007) Article DOI: 10.1042/BJ20070797 BindingDB Entry DOI: 10.7270/Q27082B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26185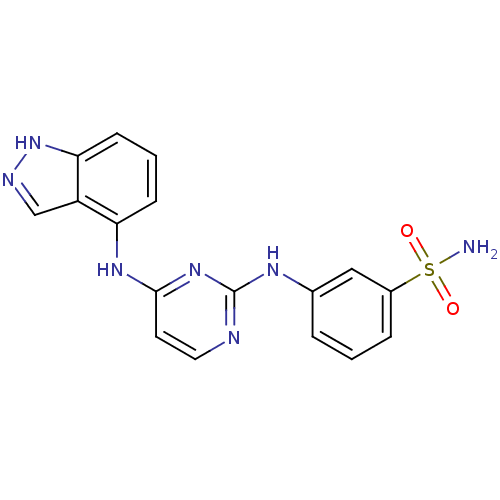 (2,4-dianilino pyrimidine, 42 | 3-{[4-(1H-indazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM25116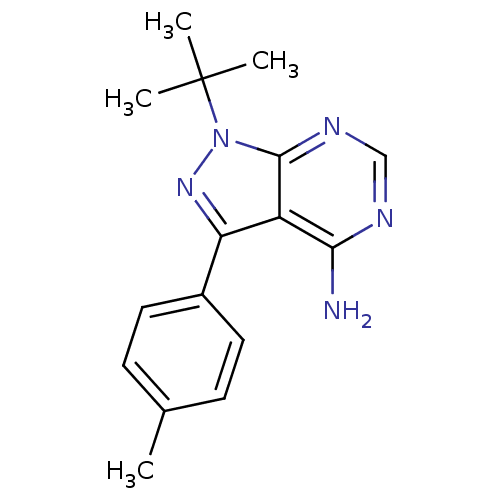 (1-tert-butyl-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of Lck in the presence of 50uM ATP | Biochem J 408: 297-315 (2007) Article DOI: 10.1042/BJ20070797 BindingDB Entry DOI: 10.7270/Q27082B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26152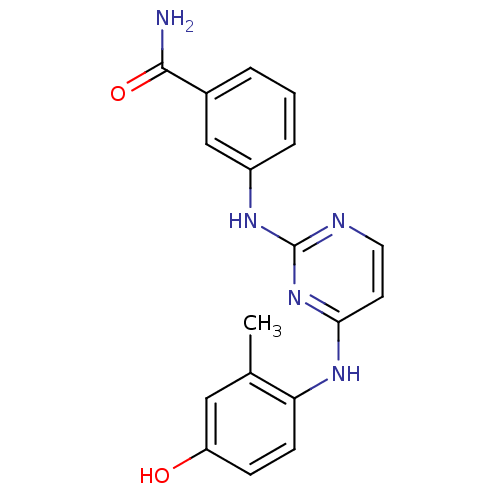 (2,4-dianilino pyrimidine, 9 | 3-({4-[(4-hydroxy-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26149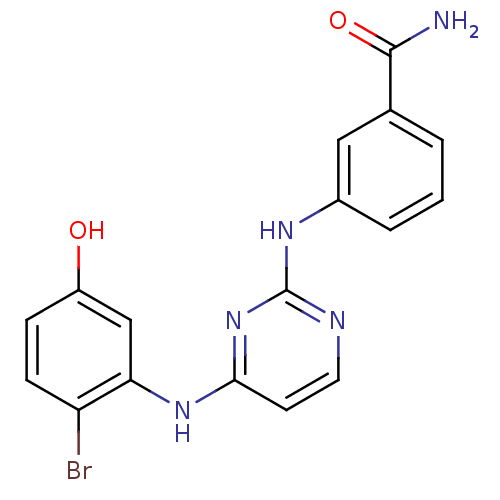 (2,4-dianilino pyrimidine, 6 | 3-({4-[(2-bromo-5-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14953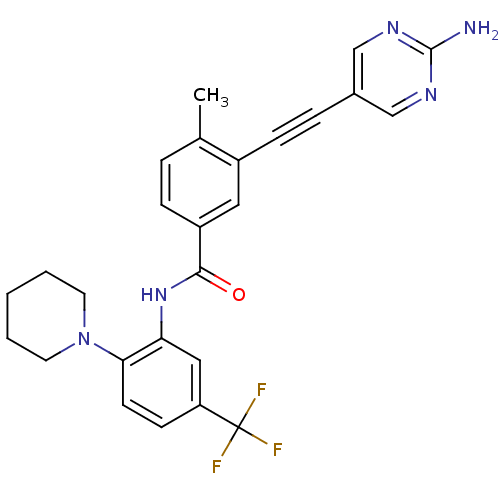 (3-(2-(2-Aminopyrimidin-5-yl)ethynyl)-4-methyl-N-(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26167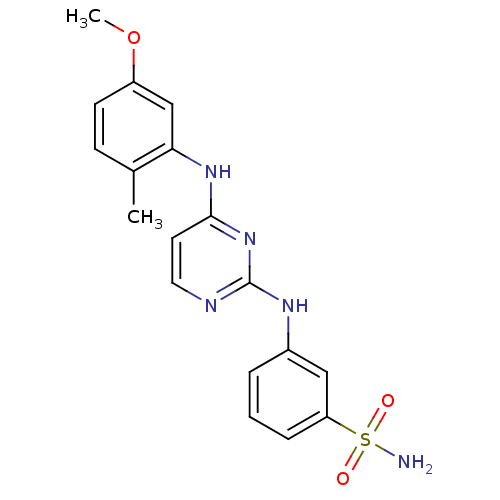 (2,4-dianilino pyrimidine, 24 | 3-({4-[(5-methoxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451789 (US10710980, Example 21 | US10947218, Example 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26182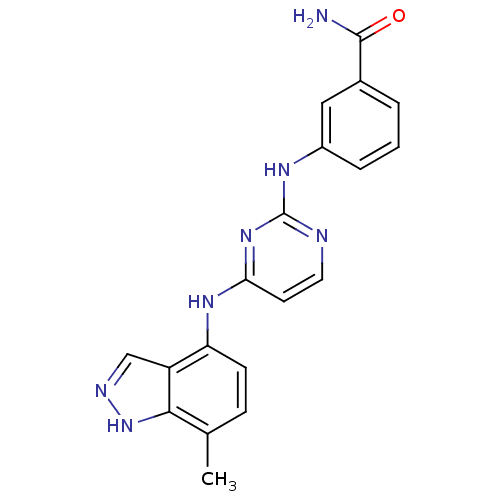 (2,4-dianilino pyrimidine, 39 | 3-({4-[(7-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451789 (US10710980, Example 21 | US10947218, Example 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM25191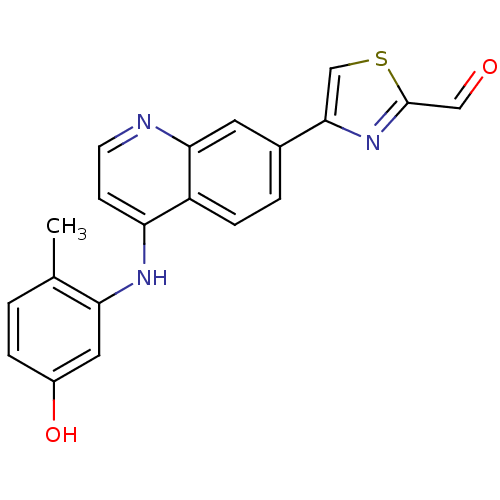 (4-{4-[(5-hydroxy-2-methylphenyl)amino]quinolin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 20 | -10.4 | 59 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 18: 318-23 (2008) Article DOI: 10.1016/j.bmcl.2007.10.076 BindingDB Entry DOI: 10.7270/Q2KP80G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM25191 (4-{4-[(5-hydroxy-2-methylphenyl)amino]quinolin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14952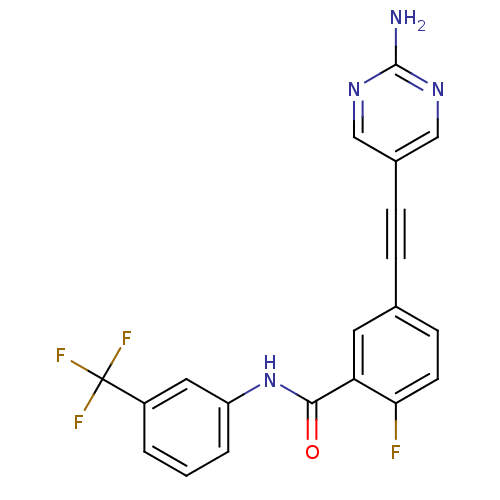 (5-(2-(2-Aminopyrimidin-5-yl)ethynyl)-2-fluoro-N-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13242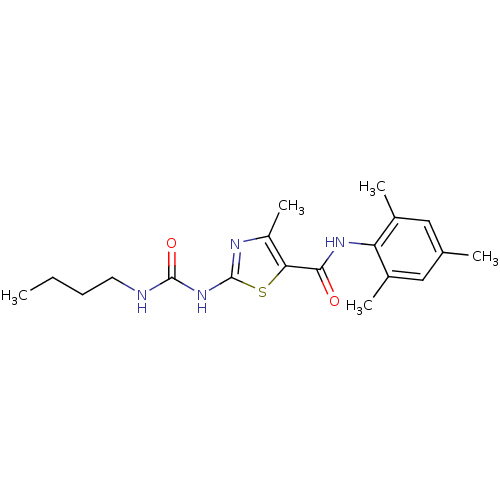 (2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13242 (2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26147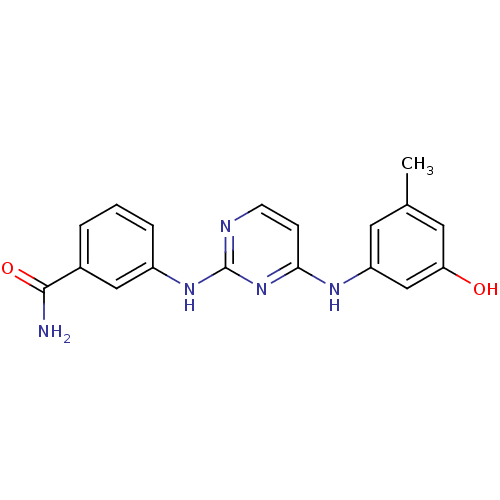 (2,4-dianilino pyrimidine, 4 | 3-({4-[(3-hydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451769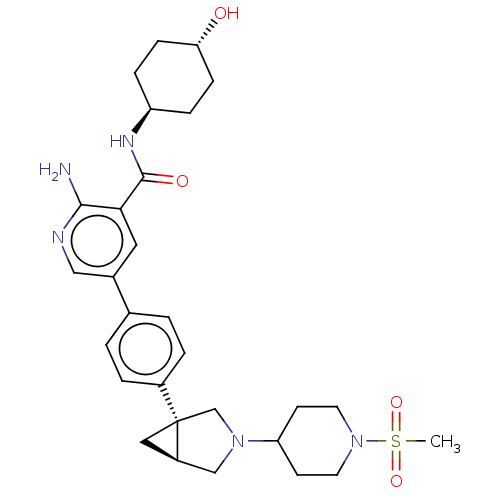 (US10710980, Example 1 | US10947218, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451769 (US10710980, Example 1 | US10947218, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451773 (US10710980, Example 5 | US10947218, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451773 (US10710980, Example 5 | US10947218, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26156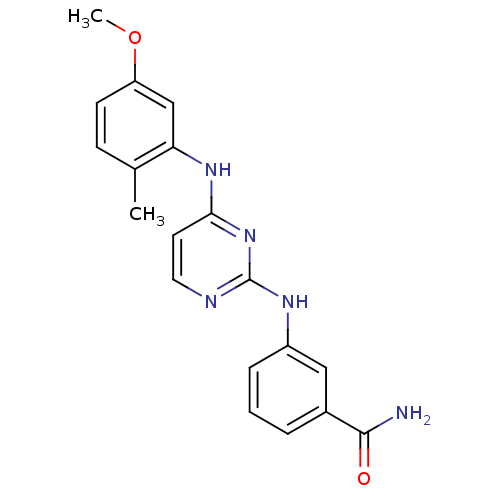 (2,4-dianilino pyrimidine, 13 | 3-({4-[(5-methoxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451778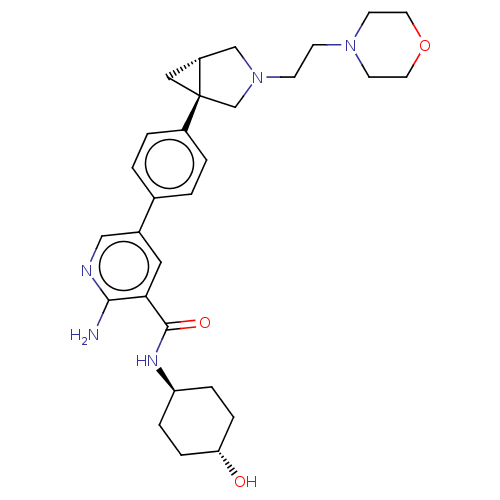 (US10710980, Example 10 | US10947218, Example 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26151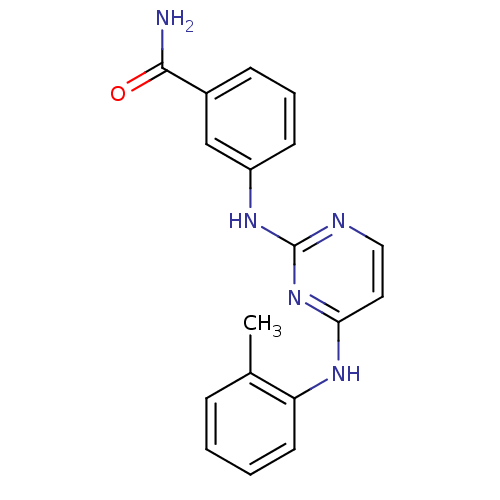 (2,4-dianilino pyrimidine, 8 | 3-({4-[(2-methylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451778 (US10710980, Example 10 | US10947218, Example 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13241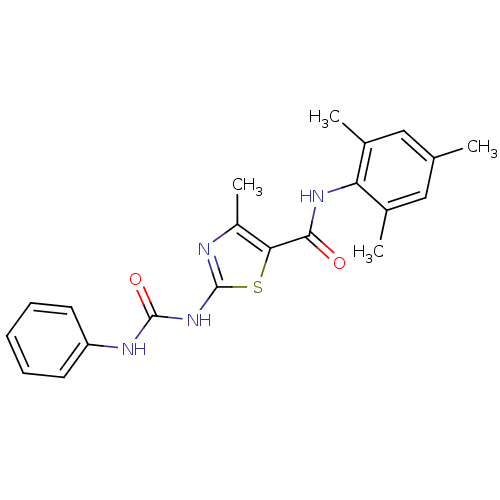 (4-methyl-2-[(phenylcarbamoyl)amino]-N-(2,4,6-trime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451770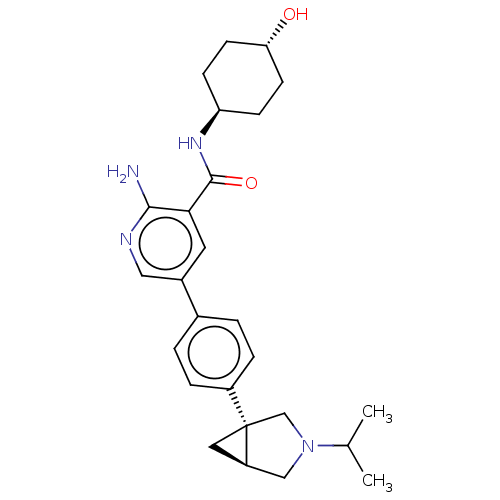 (US10710980, Example 2 | US10947218, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451770 (US10710980, Example 2 | US10947218, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26157 (2,4-dianilino pyrimidine, 14 | 3-({4-[(5-amino-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13244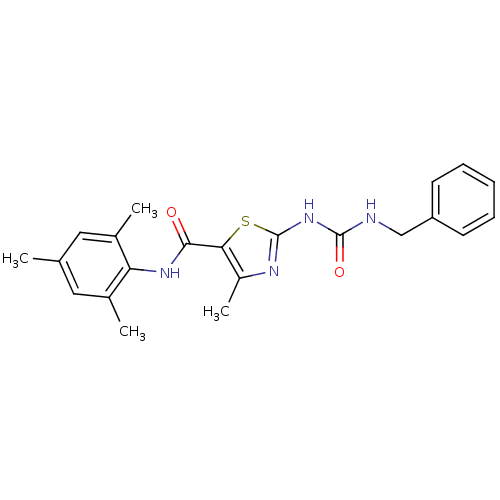 (2-[(benzylcarbamoyl)amino]-4-methyl-N-(2,4,6-trime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451805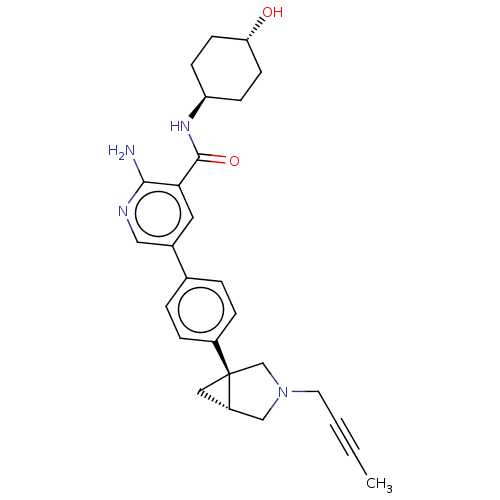 (US10710980, Example 37 | US10947218, Example 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451805 (US10710980, Example 37 | US10947218, Example 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26153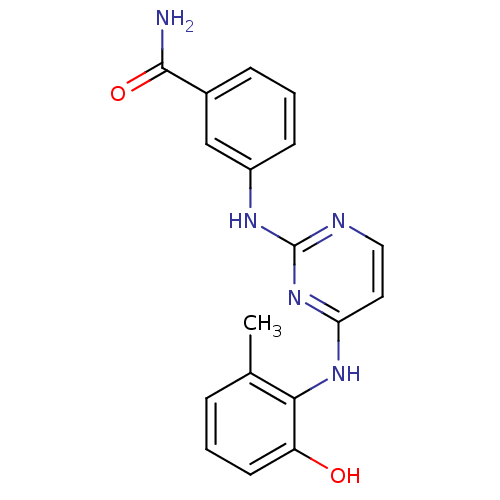 (2,4-dianilino pyrimidine, 10 | 3-({4-[(2-hydroxy-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM26179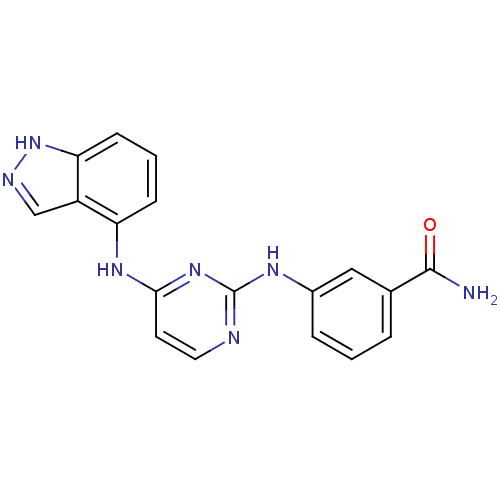 (2,4-dianilino pyrimidine, 36 | 3-{[4-(1H-indazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Lck activity was assessed using a TR-FRET assay in a 384-well plate format. The degree of phosphorylation of Biotinylated substrate was measured usin... | Bioorg Med Chem Lett 17: 4363-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.029 BindingDB Entry DOI: 10.7270/Q21R6NTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451787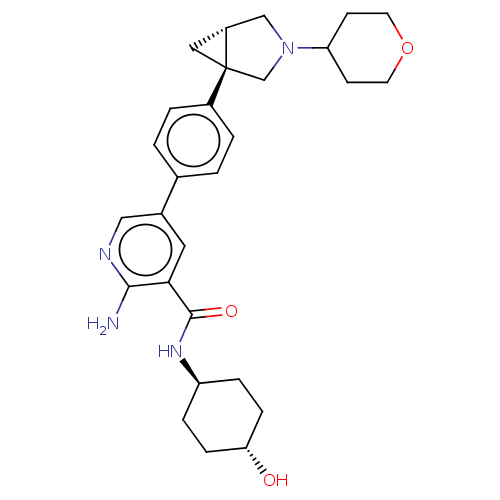 (US10710980, Example 19 | US10947218, Example 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451787 (US10710980, Example 19 | US10947218, Example 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451785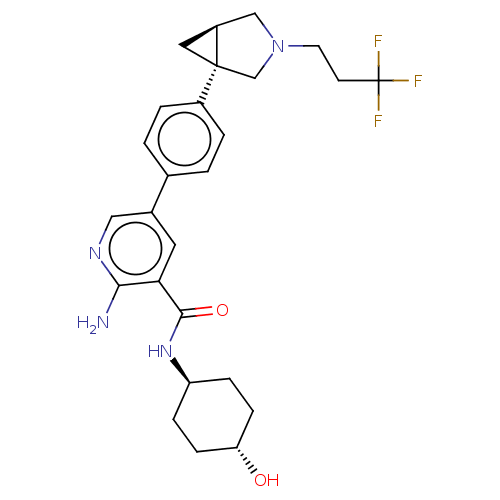 (US10710980, Example 17 | US10947218, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451785 (US10710980, Example 17 | US10947218, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 348 total ) | Next | Last >> |