 Found 10304 hits of ic50 for UniProtKB: P42336
Found 10304 hits of ic50 for UniProtKB: P42336 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089328
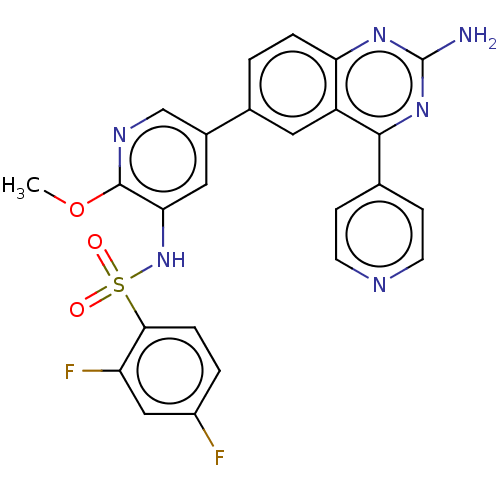
(CHEMBL3577914)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nc(N)nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H18F2N6O3S/c1-36-24-21(33-37(34,35)22-5-3-17(26)12-19(22)27)11-16(13-30-24)15-2-4-20-18(10-15)23(32-25(28)31-20)14-6-8-29-9-7-14/h2-13,33H,1H3,(H2,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089318
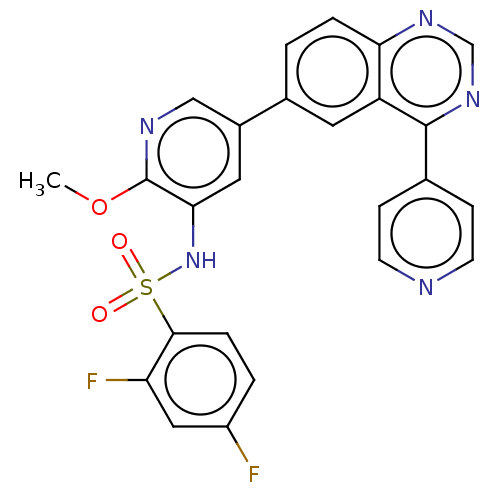
(CHEMBL3577911)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncnc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-22(32-36(33,34)23-5-3-18(26)12-20(23)27)11-17(13-29-25)16-2-4-21-19(10-16)24(31-14-30-21)15-6-8-28-9-7-15/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089321

(CHEMBL3577908)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccncc3)c2c1 Show InChI InChI=1S/C26H18F2N4O3S/c1-35-26-24(32-36(33,34)25-5-3-19(27)14-22(25)28)13-18(15-31-26)17-2-4-23-21(12-17)20(8-11-30-23)16-6-9-29-10-7-16/h2-15,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112904
BindingDB Entry DOI: 10.7270/Q2XP791Q |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50204093
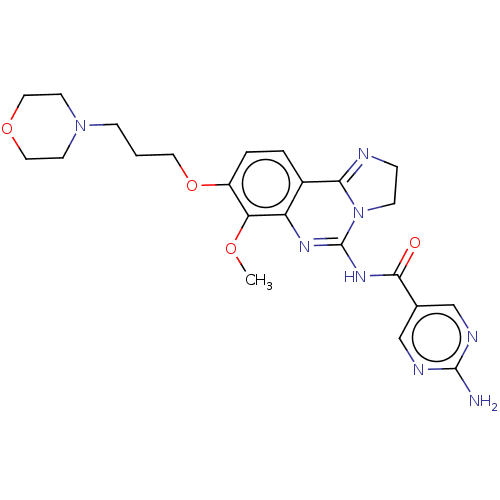
(BAY 80-6946 | BAY-80-6946 | Copanlisib)Show SMILES COc1c(OCCCN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cnc(N)nc3)=Nc12 |c:35,t:18| Show InChI InChI=1S/C23H28N8O4/c1-33-19-17(35-10-2-6-30-8-11-34-12-9-30)4-3-16-18(19)28-23(31-7-5-25-20(16)31)29-21(32)15-13-26-22(24)27-14-15/h3-4,13-14H,2,5-12H2,1H3,(H2,24,26,27)(H,28,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50579637
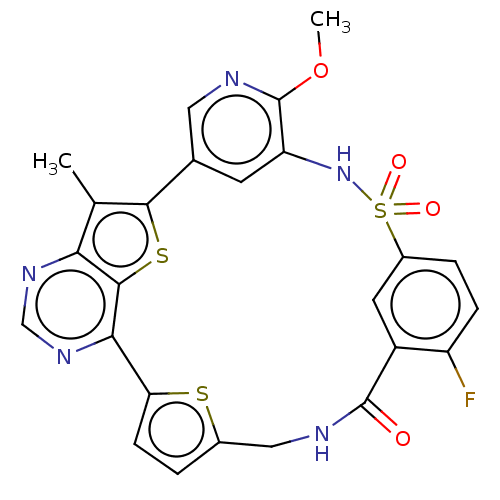
(CHEMBL4878958)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1ccc(F)c(c1)C(=O)NCc1ccc(s1)-c1ncnc3c(C)c-2sc13 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by ADP-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00412
BindingDB Entry DOI: 10.7270/Q2FX7F91 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108190
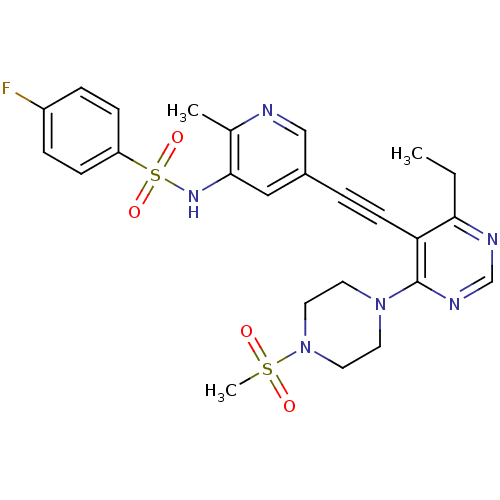
(US8598157, I-67)Show SMILES CCc1ncnc(N2CCN(CC2)S(C)(=O)=O)c1C#Cc1cnc(C)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H27FN6O4S2/c1-4-23-22(25(29-17-28-23)31-11-13-32(14-12-31)37(3,33)34)10-5-19-15-24(18(2)27-16-19)30-38(35,36)21-8-6-20(26)7-9-21/h6-9,15-17,30H,4,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089305

(CHEMBL3577927)Show SMILES Clc1ccc(s1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C20H11Cl2N5O2S3/c21-16-1-2-17(31-16)32(28,29)27-14-7-12(9-24-19(14)22)15-8-13-18(11-3-5-23-6-4-11)25-10-26-20(13)30-15/h1-10,27H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514596

(CHEMBL4455710)Show SMILES COC(=O)c1ccccc1-n1cc(CNC(=O)c2cnc3ccc(cn23)-c2cnc(OC)c(NS(=O)(=O)c3ccc(F)cc3F)c2)nn1 Show InChI InChI=1S/C31H24F2N8O6S/c1-46-30-24(38-48(44,45)27-9-8-20(32)12-23(27)33)11-19(13-36-30)18-7-10-28-34-15-26(40(28)16-18)29(42)35-14-21-17-41(39-37-21)25-6-4-3-5-22(25)31(43)47-2/h3-13,15-17,38H,14H2,1-2H3,(H,35,42) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514593

(CHEMBL4470122)Show SMILES COCCc1nnc(o1)-c1cnc2ccc(cn12)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C24H20F2N6O5S/c1-35-8-7-22-29-30-24(37-22)19-12-27-21-6-3-14(13-32(19)21)15-9-18(23(36-2)28-11-15)31-38(33,34)20-5-4-16(25)10-17(20)26/h3-6,9-13,31H,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514597

(CHEMBL4475217)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncc(-c3nnc(o3)-c3cccc(c3)C(O)=O)n2c1 Show InChI InChI=1S/C28H18F2N6O6S/c1-41-26-21(35-43(39,40)23-7-6-19(29)11-20(23)30)10-18(12-32-26)17-5-8-24-31-13-22(36(24)14-17)27-34-33-25(42-27)15-3-2-4-16(9-15)28(37)38/h2-14,35H,1H3,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514589
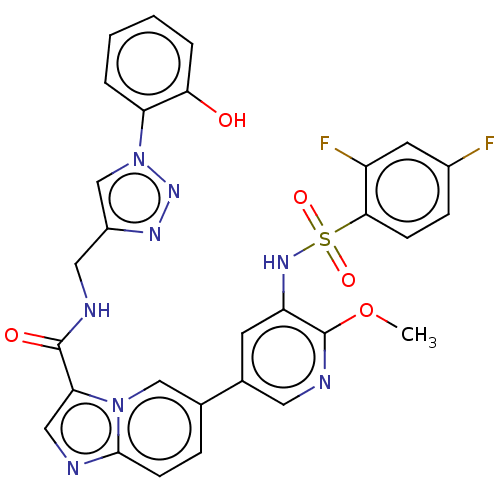
(CHEMBL4534475)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncc(C(=O)NCc3cn(nn3)-c3ccccc3O)n2c1 Show InChI InChI=1S/C29H22F2N8O5S/c1-44-29-22(36-45(42,43)26-8-7-19(30)11-21(26)31)10-18(12-34-29)17-6-9-27-32-14-24(38(27)15-17)28(41)33-13-20-16-39(37-35-20)23-4-2-3-5-25(23)40/h2-12,14-16,36,40H,13H2,1H3,(H,33,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514595
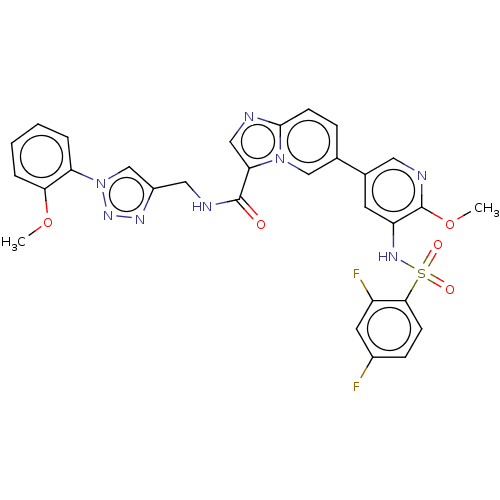
(CHEMBL4516974)Show SMILES COc1ccccc1-n1cc(CNC(=O)c2cnc3ccc(cn23)-c2cnc(OC)c(NS(=O)(=O)c3ccc(F)cc3F)c2)nn1 Show InChI InChI=1S/C30H24F2N8O5S/c1-44-26-6-4-3-5-24(26)40-17-21(36-38-40)14-34-29(41)25-15-33-28-10-7-18(16-39(25)28)19-11-23(30(45-2)35-13-19)37-46(42,43)27-9-8-20(31)12-22(27)32/h3-13,15-17,37H,14H2,1-2H3,(H,34,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514590

(CHEMBL4440406)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncc(-c3nnc(CCN4CCN(C)CC4)o3)n2c1 Show InChI InChI=1S/C28H28F2N8O4S/c1-36-9-11-37(12-10-36)8-7-26-33-34-28(42-26)23-16-31-25-6-3-18(17-38(23)25)19-13-22(27(41-2)32-15-19)35-43(39,40)24-5-4-20(29)14-21(24)30/h3-6,13-17,35H,7-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50546014

(CHEMBL4780201)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncnc(Nc3cnn(CCO)c3)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using lipid as substrate incubated for 15 mins followed by substrate addition and measured after 60 mins ADP... |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.04.024
BindingDB Entry DOI: 10.7270/Q2T1577B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108193

(US8598157, I-70)Show SMILES C[C@H]1COCCN1c1ncnc(C)c1C#Cc1cnc(C)c(NS(=O)(=O)c2ccccc2)c1 |r| Show InChI InChI=1S/C24H25N5O3S/c1-17-15-32-12-11-29(17)24-22(18(2)26-16-27-24)10-9-20-13-23(19(3)25-14-20)28-33(30,31)21-7-5-4-6-8-21/h4-8,13-14,16-17,28H,11-12,15H2,1-3H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320098
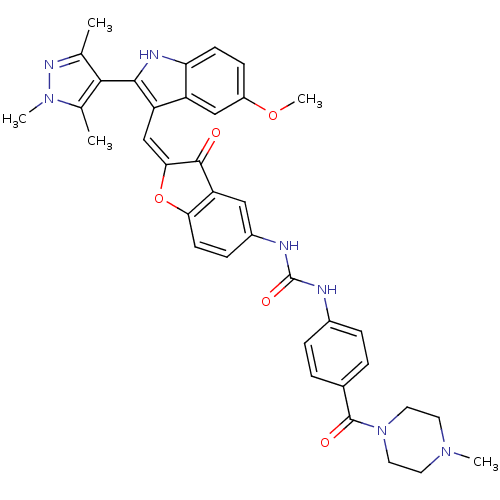
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N5CCN(C)CC5)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(-2.98,-34.6,;-2.98,-36.14,;-1.64,-36.92,;-1.65,-38.46,;-.31,-39.23,;1.02,-38.45,;2.49,-38.93,;3.4,-37.68,;2.49,-36.43,;3.09,-35.01,;2.32,-33.67,;2.95,-32.27,;1.8,-31.25,;1.8,-29.71,;.47,-28.94,;-.86,-29.72,;-2.2,-28.95,;-2.21,-27.41,;-.87,-26.64,;-3.55,-26.65,;-3.56,-25.12,;-2.23,-24.34,;-2.24,-22.81,;-3.57,-22.05,;-4.9,-22.84,;-4.88,-24.36,;-3.58,-20.52,;-2.26,-19.74,;-4.92,-19.76,;-4.92,-18.23,;-6.25,-17.48,;-7.57,-18.25,;-8.9,-17.49,;-7.56,-19.78,;-6.23,-20.55,;-.85,-31.25,;.47,-32.01,;.79,-33.52,;-.24,-34.67,;1.02,-36.91,;-.31,-36.14,;4.94,-37.68,;5.84,-38.93,;5.36,-40.39,;7.3,-38.45,;7.3,-36.91,;8.55,-36,;5.84,-36.43,;5.36,-34.96,)| Show InChI InChI=1S/C37H37N7O5/c1-21-33(22(2)43(4)41-21)34-28(27-19-26(48-5)11-12-30(27)40-34)20-32-35(45)29-18-25(10-13-31(29)49-32)39-37(47)38-24-8-6-23(7-9-24)36(46)44-16-14-42(3)15-17-44/h6-13,18-20,40H,14-17H2,1-5H3,(H2,38,39,47)/b32-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320101
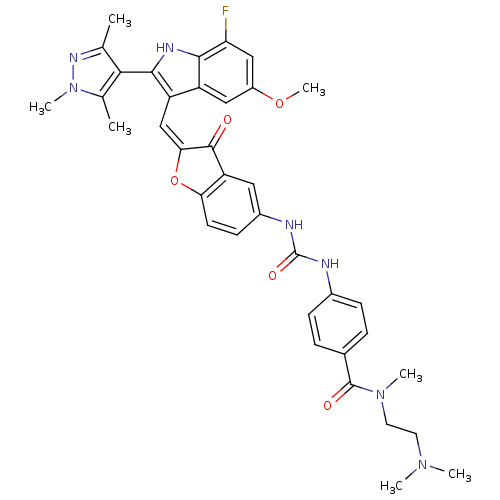
(CHEMBL1082621 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1cc(F)c2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(16.64,-8.95,;16.64,-10.49,;17.98,-11.27,;17.98,-12.81,;19.32,-13.58,;19.32,-15.12,;20.65,-12.81,;22.12,-13.28,;23.02,-12.03,;22.12,-10.78,;22.71,-9.36,;21.95,-8.02,;22.57,-6.62,;21.42,-5.6,;21.42,-4.06,;20.1,-3.29,;18.76,-4.07,;17.42,-3.3,;17.42,-1.76,;18.75,-.99,;16.08,-1,;16.07,.53,;17.4,1.31,;17.39,2.84,;16.05,3.6,;14.73,2.81,;14.74,1.29,;16.04,5.13,;17.36,5.91,;14.71,5.89,;14.69,7.42,;13.38,5.11,;12.05,5.87,;10.73,5.09,;9.39,5.85,;10.74,3.56,;18.77,-5.6,;20.1,-6.36,;20.42,-7.87,;19.38,-9.02,;20.65,-11.26,;19.31,-10.49,;24.56,-12.03,;25.46,-13.28,;24.99,-14.74,;26.93,-12.8,;26.93,-11.26,;28.18,-10.35,;25.46,-10.78,;24.99,-9.32,)| Show InChI InChI=1S/C37H38FN7O5/c1-20-32(21(2)45(6)42-20)34-27(26-17-25(49-7)18-29(38)33(26)41-34)19-31-35(46)28-16-24(12-13-30(28)50-31)40-37(48)39-23-10-8-22(9-11-23)36(47)44(5)15-14-43(3)4/h8-13,16-19,41H,14-15H2,1-7H3,(H2,39,40,48)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50349619
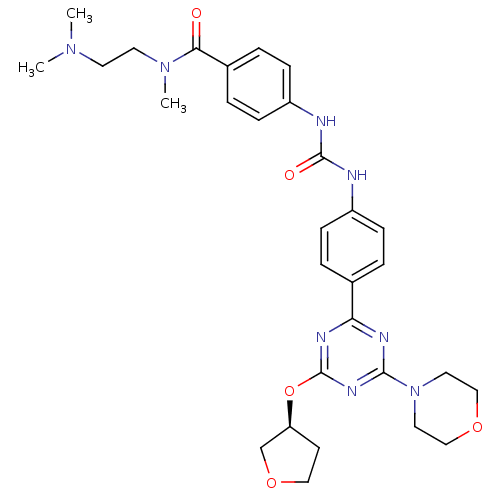
(CHEMBL1808977)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(O[C@H]3CCOC3)nc(n2)N2CCOCC2)cc1 |r| Show InChI InChI=1S/C30H38N8O5/c1-36(2)13-14-37(3)27(39)22-6-10-24(11-7-22)32-29(40)31-23-8-4-21(5-9-23)26-33-28(38-15-18-41-19-16-38)35-30(34-26)43-25-12-17-42-20-25/h4-11,25H,12-20H2,1-3H3,(H2,31,32,40)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320097
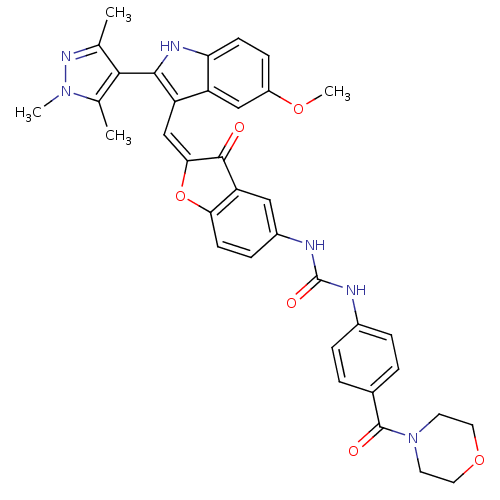
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N5CCOCC5)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(-2.53,-10.46,;-2.53,-12,;-1.2,-12.77,;-1.2,-14.32,;.14,-15.09,;1.47,-14.31,;2.94,-14.78,;3.85,-13.53,;2.94,-12.28,;3.54,-10.86,;2.77,-9.53,;3.4,-8.12,;2.25,-7.1,;2.25,-5.57,;.92,-4.79,;-.41,-5.57,;-1.75,-4.8,;-1.76,-3.26,;-.43,-2.49,;-3.1,-2.5,;-3.11,-.97,;-1.78,-.2,;-1.79,1.34,;-3.12,2.1,;-4.45,1.31,;-4.44,-.22,;-3.14,3.63,;-1.81,4.41,;-4.47,4.39,;-4.48,5.92,;-5.81,6.68,;-7.13,5.91,;-7.13,4.37,;-5.79,3.61,;-.41,-7.11,;.92,-7.86,;1.24,-9.37,;.21,-10.52,;1.47,-12.77,;.14,-12,;5.39,-13.53,;6.29,-14.78,;5.81,-16.25,;7.75,-14.31,;7.75,-12.77,;9,-11.86,;6.29,-12.29,;5.81,-10.82,)| Show InChI InChI=1S/C36H34N6O6/c1-20-32(21(2)41(3)40-20)33-27(26-18-25(46-4)10-11-29(26)39-33)19-31-34(43)28-17-24(9-12-30(28)48-31)38-36(45)37-23-7-5-22(6-8-23)35(44)42-13-15-47-16-14-42/h5-12,17-19,39H,13-16H2,1-4H3,(H2,37,38,45)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514588

(CHEMBL4439080)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(Cl)cc1F)-c1ccc2ncc(-c3nnc(CCN4CCN(C)CC4)o3)n2c1 Show InChI InChI=1S/C28H28ClFN8O4S/c1-36-9-11-37(12-10-36)8-7-26-33-34-28(42-26)23-16-31-25-6-3-18(17-38(23)25)19-13-22(27(41-2)32-15-19)35-43(39,40)24-5-4-20(29)14-21(24)30/h3-6,13-17,35H,7-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514591
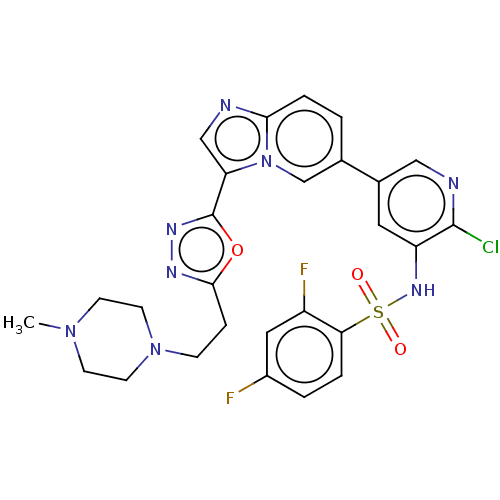
(CHEMBL4577573)Show SMILES CN1CCN(CCc2nnc(o2)-c2cnc3ccc(cn23)-c2cnc(Cl)c(NS(=O)(=O)c3ccc(F)cc3F)c2)CC1 Show InChI InChI=1S/C27H25ClF2N8O3S/c1-36-8-10-37(11-9-36)7-6-25-33-34-27(41-25)22-15-31-24-5-2-17(16-38(22)24)18-12-21(26(28)32-14-18)35-42(39,40)23-4-3-19(29)13-20(23)30/h2-5,12-16,35H,6-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089303
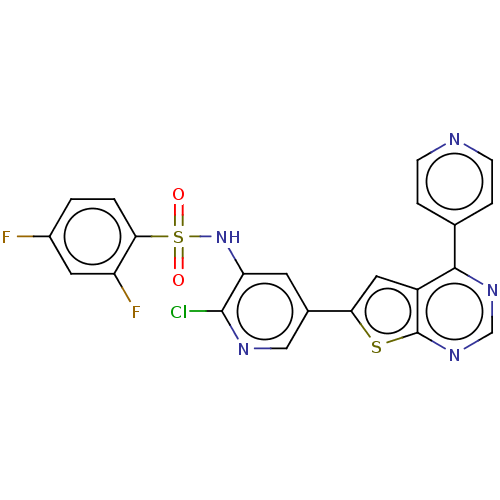
(CHEMBL3577925)Show SMILES Fc1ccc(c(F)c1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H12ClF2N5O2S2/c23-21-17(30-34(31,32)19-2-1-14(24)8-16(19)25)7-13(10-27-21)18-9-15-20(12-3-5-26-6-4-12)28-11-29-22(15)33-18/h1-11,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50579634
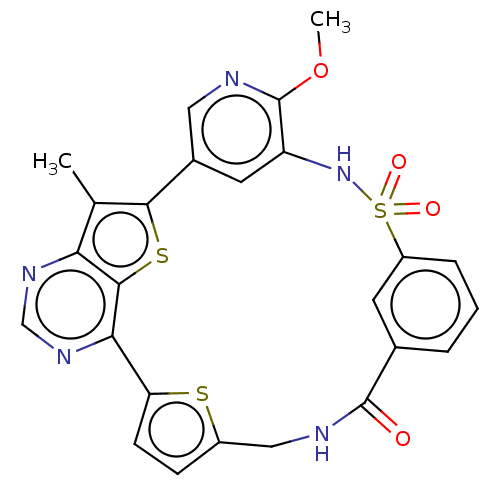
(CHEMBL4860369)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCc1ccc(s1)-c1ncnc3c(C)c-2sc13 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by ADP-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00412
BindingDB Entry DOI: 10.7270/Q2FX7F91 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514599

(CHEMBL4437362)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncc(-c3nnc(C)o3)n2c1 Show InChI InChI=1S/C22H16F2N6O4S/c1-12-27-28-22(34-12)18-10-25-20-6-3-13(11-30(18)20)14-7-17(21(33-2)26-9-14)29-35(31,32)19-5-4-15(23)8-16(19)24/h3-11,29H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50243194
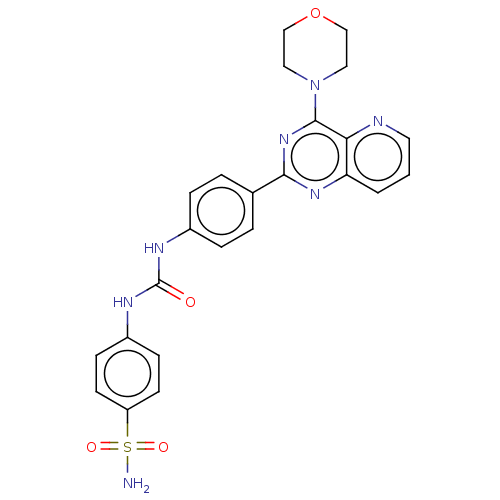
(CHEMBL4097194)Show SMILES NS(=O)(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ncccc3n2)cc1 Show InChI InChI=1S/C24H23N7O4S/c25-36(33,34)19-9-7-18(8-10-19)28-24(32)27-17-5-3-16(4-6-17)22-29-20-2-1-11-26-21(20)23(30-22)31-12-14-35-15-13-31/h1-11H,12-15H2,(H2,25,33,34)(H2,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human PI3K p110alpha/p85alpha using PIP2 as substrate preincubated for 10 mins followed by ATP addition measured after 30 m... |
Bioorg Med Chem Lett 27: 3117-3122 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.044
BindingDB Entry DOI: 10.7270/Q2Z89FTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514592

(CHEMBL4580837)Show SMILES CCc1nnc(o1)-c1cnc2ccc(cn12)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C23H18F2N6O4S/c1-3-21-28-29-23(35-21)18-11-26-20-7-4-13(12-31(18)20)14-8-17(22(34-2)27-10-14)30-36(32,33)19-6-5-15(24)9-16(19)25/h4-12,30H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25041

(N'-[(1E)-{6-cyanoimidazo[1,2-a]pyridin-3-yl}methyl...)Show SMILES CN(\N=C\c1cnc2ccc(cn12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C17H14N6O4S/c1-12-3-5-14(23(24)25)7-16(12)28(26,27)21(2)20-10-15-9-19-17-6-4-13(8-18)11-22(15)17/h3-7,9-11H,1-2H3/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089329
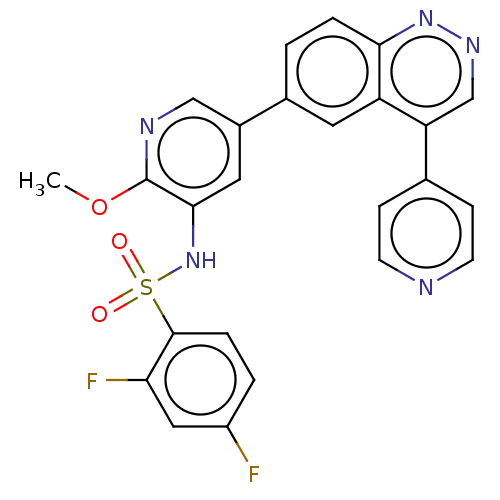
(CHEMBL3577913)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nncc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)16-2-4-22-19(10-16)20(14-30-31-22)15-6-8-28-9-7-15/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108163
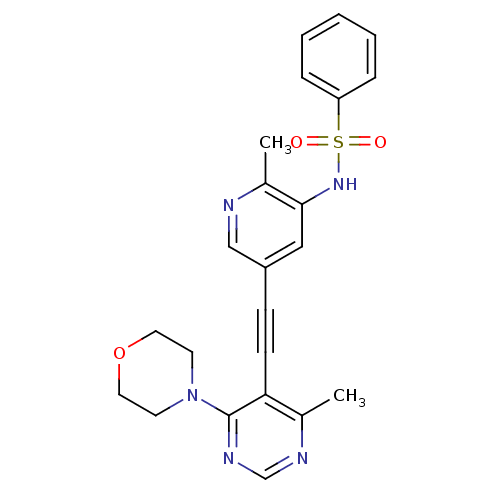
(US8598157, I-40)Show SMILES Cc1ncc(cc1NS(=O)(=O)c1ccccc1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C23H23N5O3S/c1-17-21(23(26-16-25-17)28-10-12-31-13-11-28)9-8-19-14-22(18(2)24-15-19)27-32(29,30)20-6-4-3-5-7-20/h3-7,14-16,27H,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514601
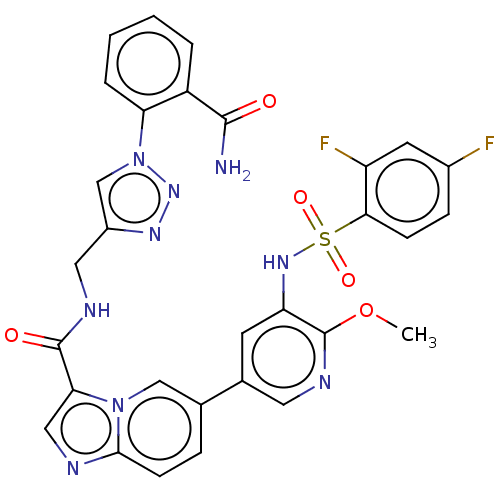
(CHEMBL4444638)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncc(C(=O)NCc3cn(nn3)-c3ccccc3C(N)=O)n2c1 Show InChI InChI=1S/C30H23F2N9O5S/c1-46-30-23(38-47(44,45)26-8-7-19(31)11-22(26)32)10-18(12-36-30)17-6-9-27-34-14-25(40(27)15-17)29(43)35-13-20-16-41(39-37-20)24-5-3-2-4-21(24)28(33)42/h2-12,14-16,38H,13H2,1H3,(H2,33,42)(H,35,43) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514602

(CHEMBL4449613)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(cc1)[N+]([O-])=O)-c1ccc2ncc(-c3nnc(CCN4CCN(C)CC4)o3)n2c1 Show InChI InChI=1S/C28H29N9O6S/c1-34-11-13-35(14-12-34)10-9-26-31-32-28(43-26)24-17-29-25-8-3-19(18-36(24)25)20-15-23(27(42-2)30-16-20)33-44(40,41)22-6-4-21(5-7-22)37(38)39/h3-8,15-18,33H,9-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kalpha using PIP2 as substrate incubated for 1 hr by kinase-glo luminescent assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113249
BindingDB Entry DOI: 10.7270/Q2PC3641 |
More data for this
Ligand-Target Pair | |
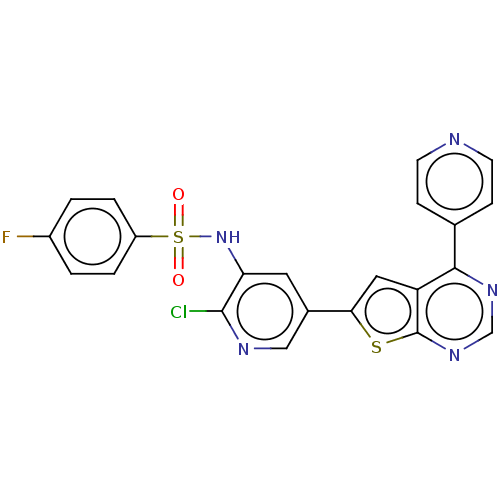
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089304
(CHEMBL3577926)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H13ClFN5O2S2/c23-21-18(29-33(30,31)16-3-1-15(24)2-4-16)9-14(11-26-21)19-10-17-20(13-5-7-25-8-6-13)27-12-28-22(17)32-19/h1-12,29H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
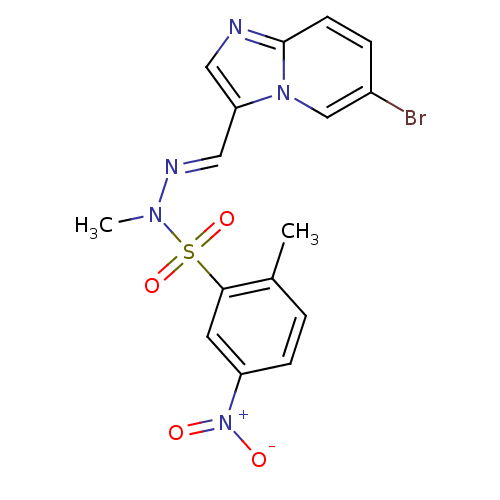
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25036
(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108207

(US8598157, I-84)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(Cl)cc1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C23H22ClN5O4S/c1-16-20(22(27-15-26-16)29-9-11-33-12-10-29)8-3-17-13-21(23(32-2)25-14-17)28-34(30,31)19-6-4-18(24)5-7-19/h4-7,13-15,28H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320099
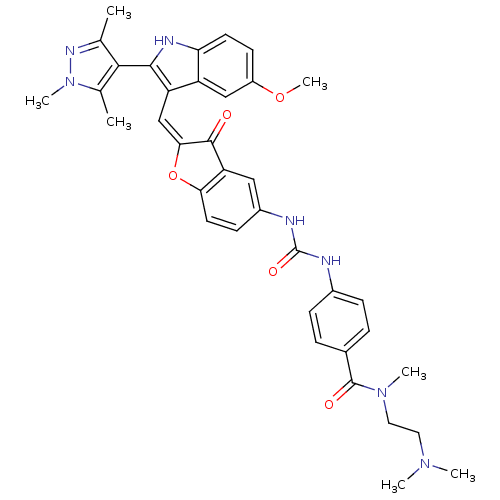
(CHEMBL1082619 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(17.28,-35.65,;17.28,-37.19,;18.62,-37.96,;18.61,-39.51,;19.95,-40.28,;21.28,-39.5,;22.75,-39.97,;23.66,-38.72,;22.75,-37.47,;23.35,-36.05,;22.58,-34.72,;23.21,-33.32,;22.06,-32.29,;22.06,-30.76,;20.73,-29.99,;19.4,-30.77,;18.06,-30,;18.05,-28.46,;19.39,-27.68,;16.71,-27.69,;16.7,-26.16,;18.03,-25.39,;18.02,-23.86,;16.69,-23.1,;15.36,-23.88,;15.38,-25.41,;16.67,-21.56,;18,-20.79,;15.34,-20.81,;15.33,-19.27,;14.02,-21.59,;12.68,-20.83,;11.36,-21.61,;10.03,-20.85,;11.38,-23.14,;19.4,-32.3,;20.73,-33.06,;21.05,-34.56,;20.02,-35.71,;21.28,-37.96,;19.95,-37.19,;25.2,-38.72,;26.1,-39.97,;25.62,-41.44,;27.56,-39.5,;27.56,-37.96,;28.81,-37.05,;26.1,-37.48,;25.62,-36.01,)| Show InChI InChI=1S/C37H39N7O5/c1-21-33(22(2)44(6)41-21)34-28(27-19-26(48-7)13-14-30(27)40-34)20-32-35(45)29-18-25(12-15-31(29)49-32)39-37(47)38-24-10-8-23(9-11-24)36(46)43(5)17-16-42(3)4/h8-15,18-20,40H,16-17H2,1-7H3,(H2,38,39,47)/b32-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50456923

(CHEMBL4213353)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncc(C(=O)NCCN3CCOCC3)n2c1 Show InChI InChI=1S/C26H26F2N6O5S/c1-38-26-21(32-40(36,37)23-4-3-19(27)13-20(23)28)12-18(14-31-26)17-2-5-24-30-15-22(34(24)16-17)25(35)29-6-7-33-8-10-39-11-9-33/h2-5,12-16,32H,6-11H2,1H3,(H,29,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using lipid as substrate incubated for 15 mins followed by substrate addition and measured after 60 mins ADP... |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.04.024
BindingDB Entry DOI: 10.7270/Q2T1577B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514598

(CHEMBL4536505)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncc(-c3cn(CCCN4CCOCC4)nn3)n2c1 Show InChI InChI=1S/C28H28F2N8O4S/c1-41-28-23(34-43(39,40)26-5-4-21(29)14-22(26)30)13-20(15-32-28)19-3-6-27-31-16-25(38(27)17-19)24-18-37(35-33-24)8-2-7-36-9-11-42-12-10-36/h3-6,13-18,34H,2,7-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50514600

(CHEMBL4441605)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncc(C(=O)NCc3cn(CCN4CCN(C)CC4)nn3)n2c1 Show InChI InChI=1S/C30H32F2N10O4S/c1-39-7-9-40(10-8-39)11-12-41-19-23(36-38-41)16-34-29(43)26-17-33-28-6-3-20(18-42(26)28)21-13-25(30(46-2)35-15-21)37-47(44,45)27-5-4-22(31)14-24(27)32/h3-6,13-15,17-19,37H,7-12,16H2,1-2H3,(H,34,43) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... |
J Med Chem 63: 3028-3046 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01736
BindingDB Entry DOI: 10.7270/Q2QV3QVS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108208
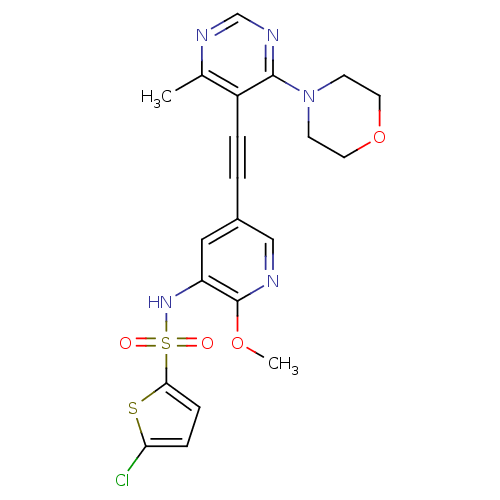
(US8598157, I-85)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(Cl)s1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C21H20ClN5O4S2/c1-14-16(20(25-13-24-14)27-7-9-31-10-8-27)4-3-15-11-17(21(30-2)23-12-15)26-33(28,29)19-6-5-18(22)32-19/h5-6,11-13,26H,7-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108209
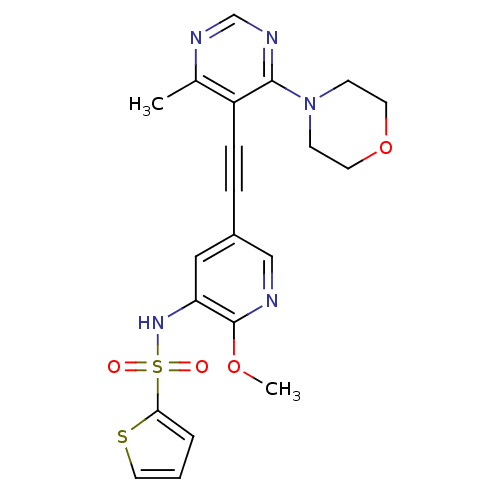
(US8598157, I-86)Show SMILES COc1ncc(cc1NS(=O)(=O)c1cccs1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C21H21N5O4S2/c1-15-17(20(24-14-23-15)26-7-9-30-10-8-26)6-5-16-12-18(21(29-2)22-13-16)25-32(27,28)19-4-3-11-31-19/h3-4,11-14,25H,7-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135
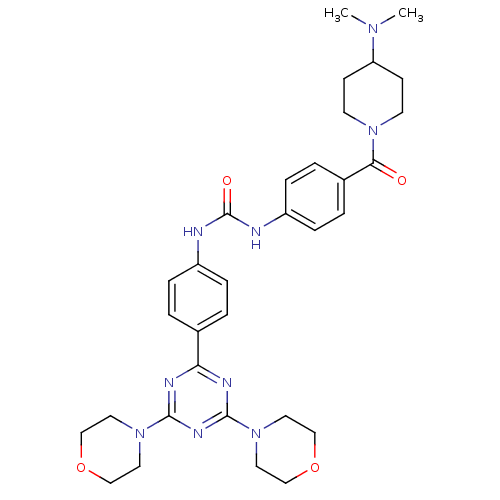
(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114039
BindingDB Entry DOI: 10.7270/Q2TT4VZR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50502319
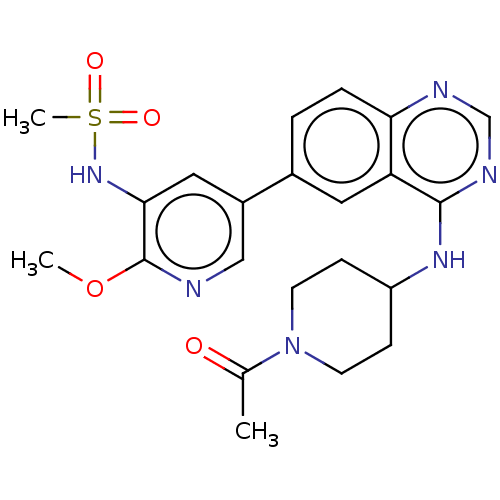
(CHEMBL4515169)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(NC3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C22H26N6O4S/c1-14(29)28-8-6-17(7-9-28)26-21-18-10-15(4-5-19(18)24-13-25-21)16-11-20(27-33(3,30)31)22(32-2)23-12-16/h4-5,10-13,17,27H,6-9H2,1-3H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of wild type human PI3K p110alpha using PIP2 as substrate by TR-FRET assay |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320095
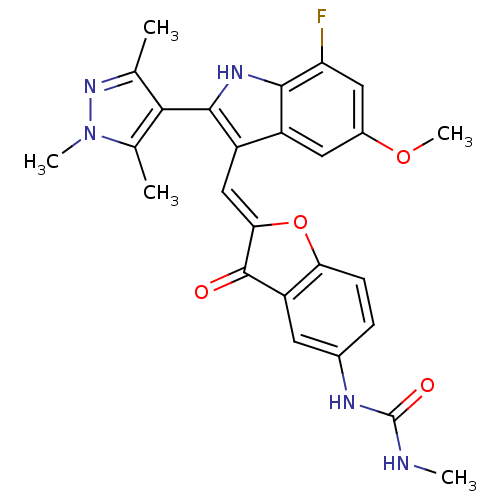
(1-(2-((7-fluoro-5-methoxy-2-(1,3,5-trimethyl-1H-py...)Show SMILES CNC(=O)Nc1ccc2O\C(=C\c3c([nH]c4c(F)cc(OC)cc34)-c3c(C)nn(C)c3C)C(=O)c2c1 |(15.41,-12.32,;16.73,-11.54,;18.07,-12.3,;19.4,-11.53,;18.07,-13.84,;19.41,-14.61,;20.75,-13.83,;22.07,-14.6,;22.07,-16.14,;23.22,-17.16,;22.59,-18.56,;23.36,-19.9,;22.76,-21.32,;23.67,-22.57,;22.77,-23.82,;21.3,-23.34,;19.96,-24.12,;19.97,-25.65,;18.63,-23.35,;18.63,-21.81,;17.29,-21.03,;17.29,-19.49,;19.96,-21.03,;21.3,-21.8,;25.21,-22.57,;26.11,-23.81,;25.63,-25.28,;27.57,-23.34,;27.57,-21.8,;28.82,-20.89,;26.11,-21.32,;25.63,-19.85,;21.06,-18.41,;20.03,-19.56,;20.75,-16.9,;19.42,-16.14,)| Show InChI InChI=1S/C26H24FN5O4/c1-12-22(13(2)32(4)31-12)24-17(16-9-15(35-5)10-19(27)23(16)30-24)11-21-25(33)18-8-14(29-26(34)28-3)6-7-20(18)36-21/h6-11,30H,1-5H3,(H2,28,29,34)/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320103
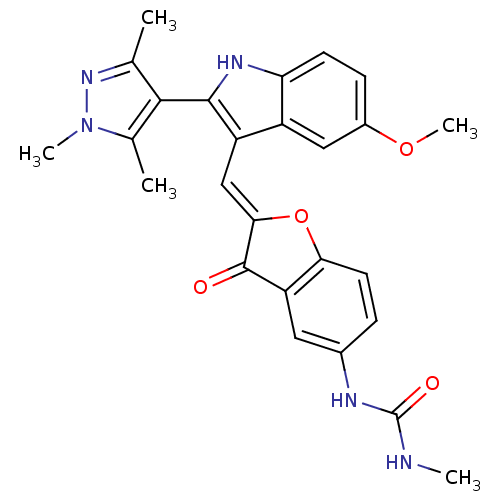
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES CNC(=O)Nc1ccc2O\C(=C\c3c([nH]c4ccc(OC)cc34)-c3c(C)nn(C)c3C)C(=O)c2c1 |(-6.21,5.21,;-4.89,5.98,;-3.55,5.22,;-2.22,5.99,;-3.55,3.68,;-2.21,2.91,;-.87,3.69,;.45,2.92,;.45,1.39,;1.6,.37,;.97,-1.04,;1.74,-2.37,;1.14,-3.79,;2.05,-5.04,;1.14,-6.29,;-.32,-5.82,;-1.66,-6.59,;-2.99,-5.82,;-2.99,-4.28,;-4.33,-3.51,;-4.33,-1.97,;-1.66,-3.51,;-.32,-4.27,;3.59,-5.04,;4.49,-6.29,;4.01,-7.75,;5.95,-5.81,;5.95,-4.27,;7.2,-3.36,;4.49,-3.79,;4.01,-2.33,;-.56,-.88,;-1.59,-2.03,;-.87,.63,;-2.2,1.38,)| Show InChI InChI=1S/C26H25N5O4/c1-13-23(14(2)31(4)30-13)24-18(17-11-16(34-5)7-8-20(17)29-24)12-22-25(32)19-10-15(28-26(33)27-3)6-9-21(19)35-22/h6-12,29H,1-5H3,(H2,27,28,33)/b22-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data