 Found 8496 hits of ic50 data for polymerid = 1622
Found 8496 hits of ic50 data for polymerid = 1622 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089328
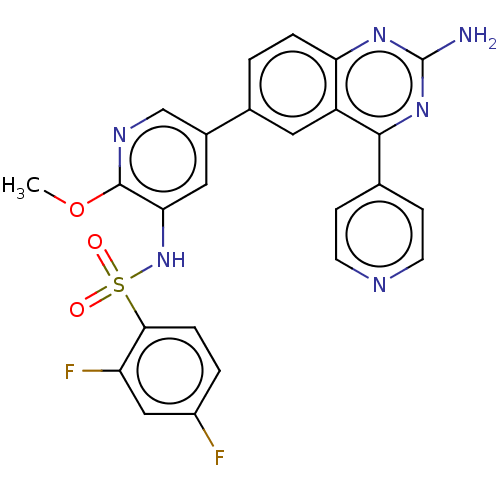
(CHEMBL3577914)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nc(N)nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H18F2N6O3S/c1-36-24-21(33-37(34,35)22-5-3-17(26)12-19(22)27)11-16(13-30-24)15-2-4-20-18(10-15)23(32-25(28)31-20)14-6-8-29-9-7-14/h2-13,33H,1H3,(H2,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089318
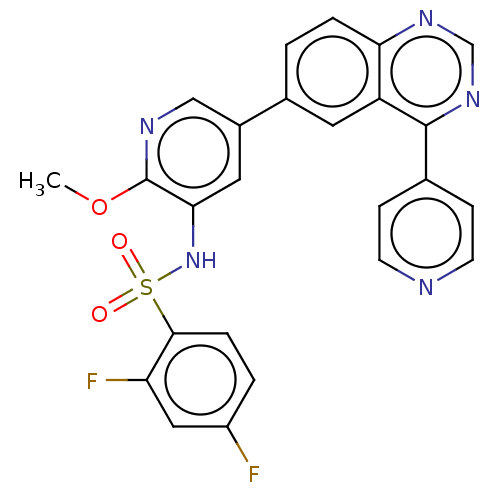
(CHEMBL3577911)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncnc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-22(32-36(33,34)23-5-3-18(26)12-20(23)27)11-17(13-29-25)16-2-4-21-19(10-16)24(31-14-30-21)15-6-8-28-9-7-15/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089321

(CHEMBL3577908)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccncc3)c2c1 Show InChI InChI=1S/C26H18F2N4O3S/c1-35-26-24(32-36(33,34)25-5-3-19(27)14-22(25)28)13-18(15-31-26)17-2-4-23-21(12-17)20(8-11-30-23)16-6-9-29-10-7-16/h2-15,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028
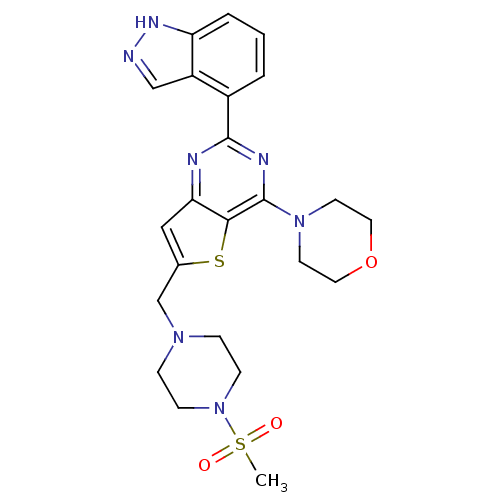
(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112904
BindingDB Entry DOI: 10.7270/Q2XP791Q |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50579637
(CHEMBL4878958)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1ccc(F)c(c1)C(=O)NCc1ccc(s1)-c1ncnc3c(C)c-2sc13 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by ADP-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00412
BindingDB Entry DOI: 10.7270/Q2FX7F91 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108190
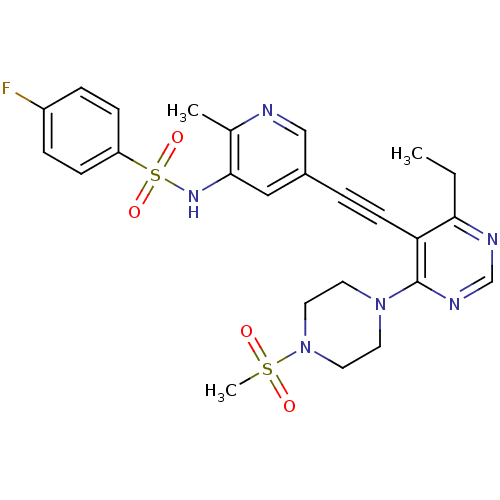
(US8598157, I-67)Show SMILES CCc1ncnc(N2CCN(CC2)S(C)(=O)=O)c1C#Cc1cnc(C)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H27FN6O4S2/c1-4-23-22(25(29-17-28-23)31-11-13-32(14-12-31)37(3,33)34)10-5-19-15-24(18(2)27-16-19)30-38(35,36)21-8-6-20(26)7-9-21/h6-9,15-17,30H,4,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089305

(CHEMBL3577927)Show SMILES Clc1ccc(s1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C20H11Cl2N5O2S3/c21-16-1-2-17(31-16)32(28,29)27-14-7-12(9-24-19(14)22)15-8-13-18(11-3-5-23-6-4-11)25-10-26-20(13)30-15/h1-10,27H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50546014

(CHEMBL4780201)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncnc(Nc3cnn(CCO)c3)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using lipid as substrate incubated for 15 mins followed by substrate addition and measured after 60 mins ADP... |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.04.024
BindingDB Entry DOI: 10.7270/Q2T1577B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320097
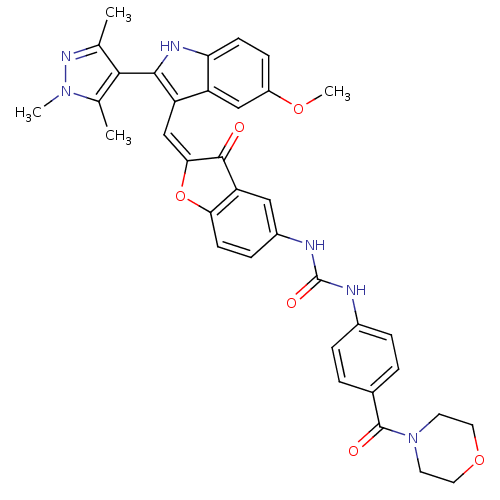
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N5CCOCC5)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(-2.53,-10.46,;-2.53,-12,;-1.2,-12.77,;-1.2,-14.32,;.14,-15.09,;1.47,-14.31,;2.94,-14.78,;3.85,-13.53,;2.94,-12.28,;3.54,-10.86,;2.77,-9.53,;3.4,-8.12,;2.25,-7.1,;2.25,-5.57,;.92,-4.79,;-.41,-5.57,;-1.75,-4.8,;-1.76,-3.26,;-.43,-2.49,;-3.1,-2.5,;-3.11,-.97,;-1.78,-.2,;-1.79,1.34,;-3.12,2.1,;-4.45,1.31,;-4.44,-.22,;-3.14,3.63,;-1.81,4.41,;-4.47,4.39,;-4.48,5.92,;-5.81,6.68,;-7.13,5.91,;-7.13,4.37,;-5.79,3.61,;-.41,-7.11,;.92,-7.86,;1.24,-9.37,;.21,-10.52,;1.47,-12.77,;.14,-12,;5.39,-13.53,;6.29,-14.78,;5.81,-16.25,;7.75,-14.31,;7.75,-12.77,;9,-11.86,;6.29,-12.29,;5.81,-10.82,)| Show InChI InChI=1S/C36H34N6O6/c1-20-32(21(2)41(3)40-20)33-27(26-18-25(46-4)10-11-29(26)39-33)19-31-34(43)28-17-24(9-12-30(28)48-31)38-36(45)37-23-7-5-22(6-8-23)35(44)42-13-15-47-16-14-42/h5-12,17-19,39H,13-16H2,1-4H3,(H2,37,38,45)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108193

(US8598157, I-70)Show SMILES C[C@H]1COCCN1c1ncnc(C)c1C#Cc1cnc(C)c(NS(=O)(=O)c2ccccc2)c1 |r| Show InChI InChI=1S/C24H25N5O3S/c1-17-15-32-12-11-29(17)24-22(18(2)26-16-27-24)10-9-20-13-23(19(3)25-14-20)28-33(30,31)21-7-5-4-6-8-21/h4-8,13-14,16-17,28H,11-12,15H2,1-3H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320101
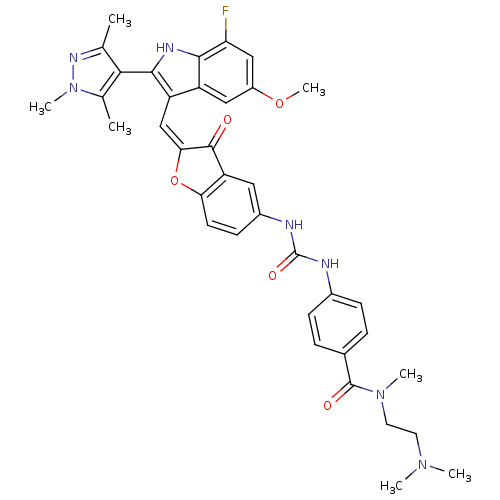
(CHEMBL1082621 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1cc(F)c2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(16.64,-8.95,;16.64,-10.49,;17.98,-11.27,;17.98,-12.81,;19.32,-13.58,;19.32,-15.12,;20.65,-12.81,;22.12,-13.28,;23.02,-12.03,;22.12,-10.78,;22.71,-9.36,;21.95,-8.02,;22.57,-6.62,;21.42,-5.6,;21.42,-4.06,;20.1,-3.29,;18.76,-4.07,;17.42,-3.3,;17.42,-1.76,;18.75,-.99,;16.08,-1,;16.07,.53,;17.4,1.31,;17.39,2.84,;16.05,3.6,;14.73,2.81,;14.74,1.29,;16.04,5.13,;17.36,5.91,;14.71,5.89,;14.69,7.42,;13.38,5.11,;12.05,5.87,;10.73,5.09,;9.39,5.85,;10.74,3.56,;18.77,-5.6,;20.1,-6.36,;20.42,-7.87,;19.38,-9.02,;20.65,-11.26,;19.31,-10.49,;24.56,-12.03,;25.46,-13.28,;24.99,-14.74,;26.93,-12.8,;26.93,-11.26,;28.18,-10.35,;25.46,-10.78,;24.99,-9.32,)| Show InChI InChI=1S/C37H38FN7O5/c1-20-32(21(2)45(6)42-20)34-27(26-17-25(49-7)18-29(38)33(26)41-34)19-31-35(46)28-16-24(12-13-30(28)50-31)40-37(48)39-23-10-8-22(9-11-23)36(47)44(5)15-14-43(3)4/h8-13,16-19,41H,14-15H2,1-7H3,(H2,39,40,48)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50349619
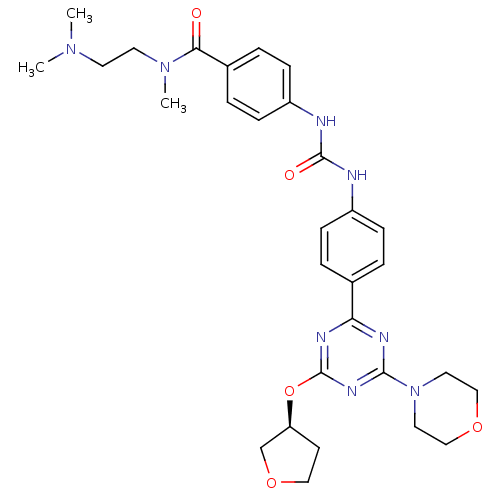
(CHEMBL1808977)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(O[C@H]3CCOC3)nc(n2)N2CCOCC2)cc1 |r| Show InChI InChI=1S/C30H38N8O5/c1-36(2)13-14-37(3)27(39)22-6-10-24(11-7-22)32-29(40)31-23-8-4-21(5-9-23)26-33-28(38-15-18-41-19-16-38)35-30(34-26)43-25-12-17-42-20-25/h4-11,25H,12-20H2,1-3H3,(H2,31,32,40)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320098
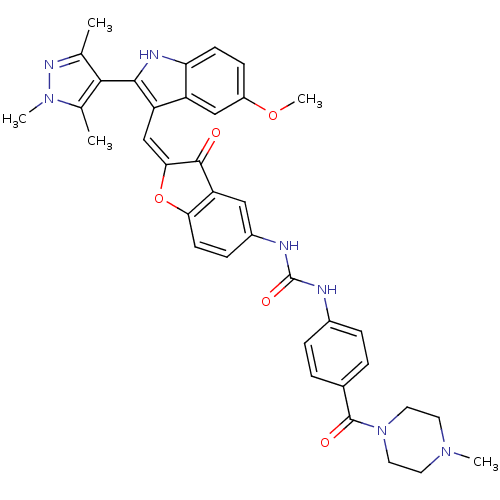
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N5CCN(C)CC5)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(-2.98,-34.6,;-2.98,-36.14,;-1.64,-36.92,;-1.65,-38.46,;-.31,-39.23,;1.02,-38.45,;2.49,-38.93,;3.4,-37.68,;2.49,-36.43,;3.09,-35.01,;2.32,-33.67,;2.95,-32.27,;1.8,-31.25,;1.8,-29.71,;.47,-28.94,;-.86,-29.72,;-2.2,-28.95,;-2.21,-27.41,;-.87,-26.64,;-3.55,-26.65,;-3.56,-25.12,;-2.23,-24.34,;-2.24,-22.81,;-3.57,-22.05,;-4.9,-22.84,;-4.88,-24.36,;-3.58,-20.52,;-2.26,-19.74,;-4.92,-19.76,;-4.92,-18.23,;-6.25,-17.48,;-7.57,-18.25,;-8.9,-17.49,;-7.56,-19.78,;-6.23,-20.55,;-.85,-31.25,;.47,-32.01,;.79,-33.52,;-.24,-34.67,;1.02,-36.91,;-.31,-36.14,;4.94,-37.68,;5.84,-38.93,;5.36,-40.39,;7.3,-38.45,;7.3,-36.91,;8.55,-36,;5.84,-36.43,;5.36,-34.96,)| Show InChI InChI=1S/C37H37N7O5/c1-21-33(22(2)43(4)41-21)34-28(27-19-26(48-5)11-12-30(27)40-34)20-32-35(45)29-18-25(10-13-31(29)49-32)39-37(47)38-24-8-6-23(7-9-24)36(46)44-16-14-42(3)15-17-44/h6-13,18-20,40H,14-17H2,1-5H3,(H2,38,39,47)/b32-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089303
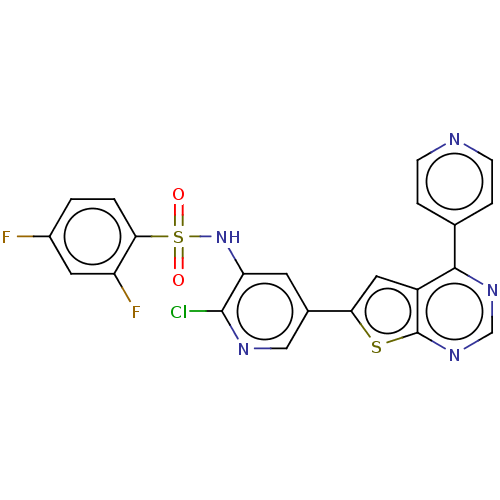
(CHEMBL3577925)Show SMILES Fc1ccc(c(F)c1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H12ClF2N5O2S2/c23-21-17(30-34(31,32)19-2-1-14(24)8-16(19)25)7-13(10-27-21)18-9-15-20(12-3-5-26-6-4-12)28-11-29-22(15)33-18/h1-11,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50579634
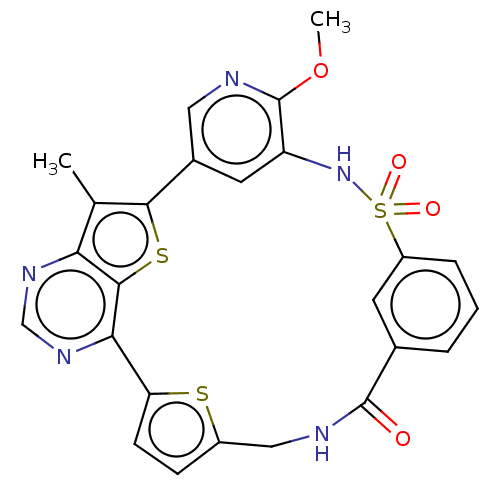
(CHEMBL4860369)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCc1ccc(s1)-c1ncnc3c(C)c-2sc13 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by ADP-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00412
BindingDB Entry DOI: 10.7270/Q2FX7F91 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25041

(N'-[(1E)-{6-cyanoimidazo[1,2-a]pyridin-3-yl}methyl...)Show SMILES CN(\N=C\c1cnc2ccc(cn12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C17H14N6O4S/c1-12-3-5-14(23(24)25)7-16(12)28(26,27)21(2)20-10-15-9-19-17-6-4-13(8-18)11-22(15)17/h3-7,9-11H,1-2H3/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089329
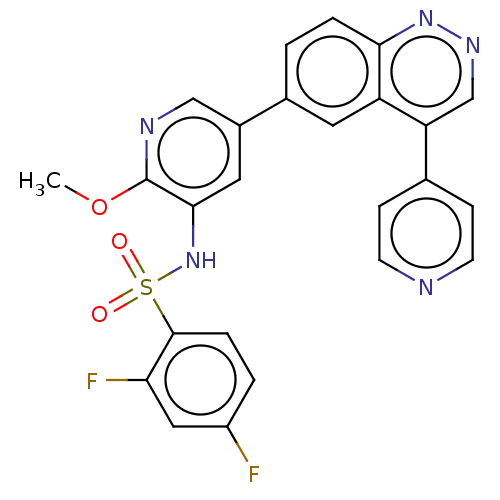
(CHEMBL3577913)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nncc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)16-2-4-22-19(10-16)20(14-30-31-22)15-6-8-28-9-7-15/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108163
(US8598157, I-40)Show SMILES Cc1ncc(cc1NS(=O)(=O)c1ccccc1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C23H23N5O3S/c1-17-21(23(26-16-25-17)28-10-12-31-13-11-28)9-8-19-14-22(18(2)24-15-19)27-32(29,30)20-6-4-3-5-7-20/h3-7,14-16,27H,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kalpha using PIP2 as substrate incubated for 1 hr by kinase-glo luminescent assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113249
BindingDB Entry DOI: 10.7270/Q2PC3641 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
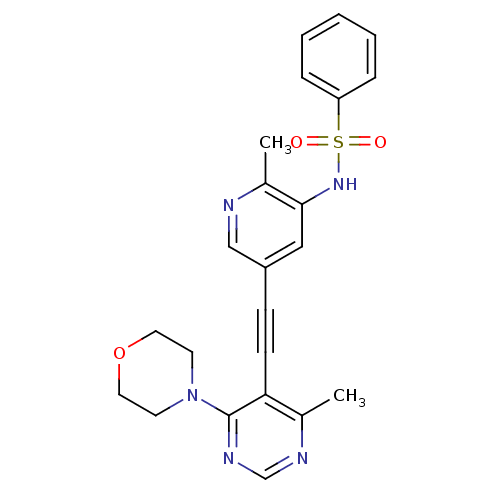
(Homo sapiens (Human)) | BDBM50320099
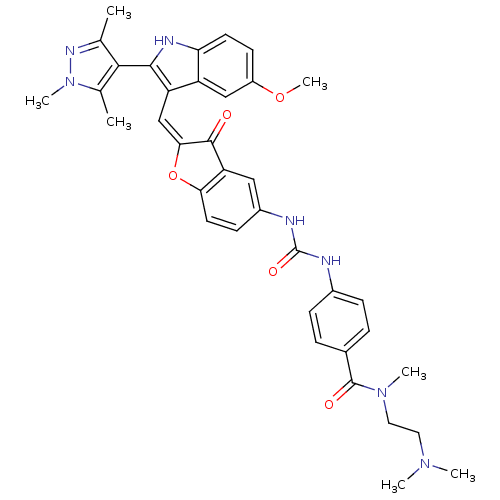
(CHEMBL1082619 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(17.28,-35.65,;17.28,-37.19,;18.62,-37.96,;18.61,-39.51,;19.95,-40.28,;21.28,-39.5,;22.75,-39.97,;23.66,-38.72,;22.75,-37.47,;23.35,-36.05,;22.58,-34.72,;23.21,-33.32,;22.06,-32.29,;22.06,-30.76,;20.73,-29.99,;19.4,-30.77,;18.06,-30,;18.05,-28.46,;19.39,-27.68,;16.71,-27.69,;16.7,-26.16,;18.03,-25.39,;18.02,-23.86,;16.69,-23.1,;15.36,-23.88,;15.38,-25.41,;16.67,-21.56,;18,-20.79,;15.34,-20.81,;15.33,-19.27,;14.02,-21.59,;12.68,-20.83,;11.36,-21.61,;10.03,-20.85,;11.38,-23.14,;19.4,-32.3,;20.73,-33.06,;21.05,-34.56,;20.02,-35.71,;21.28,-37.96,;19.95,-37.19,;25.2,-38.72,;26.1,-39.97,;25.62,-41.44,;27.56,-39.5,;27.56,-37.96,;28.81,-37.05,;26.1,-37.48,;25.62,-36.01,)| Show InChI InChI=1S/C37H39N7O5/c1-21-33(22(2)44(6)41-21)34-28(27-19-26(48-7)13-14-30(27)40-34)20-32-35(45)29-18-25(12-15-31(29)49-32)39-37(47)38-24-10-8-23(9-11-24)36(46)43(5)17-16-42(3)4/h8-15,18-20,40H,16-17H2,1-7H3,(H2,38,39,47)/b32-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089304
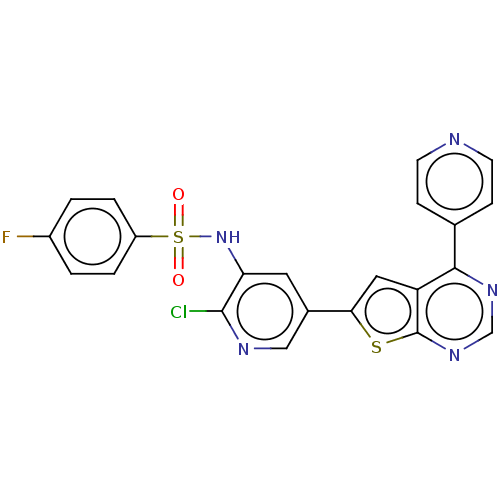
(CHEMBL3577926)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H13ClFN5O2S2/c23-21-18(29-33(30,31)16-3-1-15(24)2-4-16)9-14(11-26-21)19-10-17-20(13-5-7-25-8-6-13)27-12-28-22(17)32-19/h1-12,29H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108207

(US8598157, I-84)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(Cl)cc1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C23H22ClN5O4S/c1-16-20(22(27-15-26-16)29-9-11-33-12-10-29)8-3-17-13-21(23(32-2)25-14-17)28-34(30,31)19-6-4-18(24)5-7-19/h4-7,13-15,28H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25036
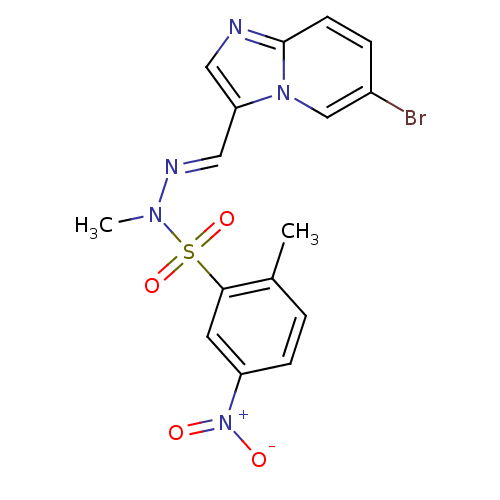
(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using lipid as substrate incubated for 15 mins followed by substrate addition and measured after 60 mins ADP... |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.04.024
BindingDB Entry DOI: 10.7270/Q2T1577B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108208
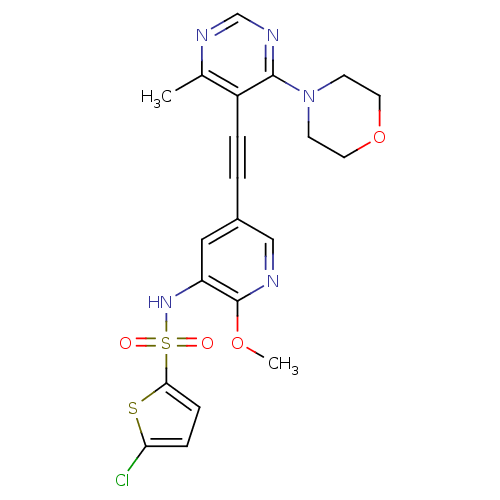
(US8598157, I-85)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(Cl)s1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C21H20ClN5O4S2/c1-14-16(20(25-13-24-14)27-7-9-31-10-8-27)4-3-15-11-17(21(30-2)23-12-15)26-33(28,29)19-6-5-18(22)32-19/h5-6,11-13,26H,7-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108209
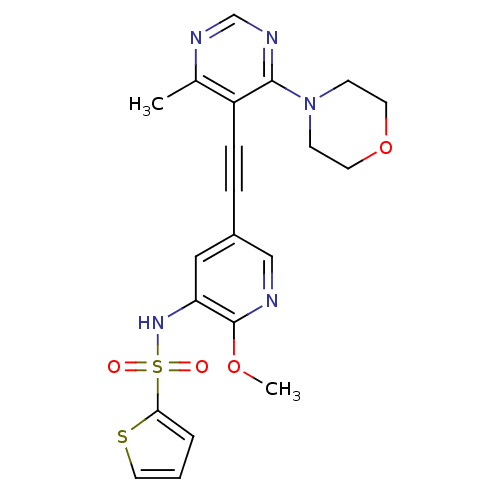
(US8598157, I-86)Show SMILES COc1ncc(cc1NS(=O)(=O)c1cccs1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C21H21N5O4S2/c1-15-17(20(24-14-23-15)26-7-9-30-10-8-26)6-5-16-12-18(21(29-2)22-13-16)25-32(27,28)19-4-3-11-31-19/h3-4,11-14,25H,7-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
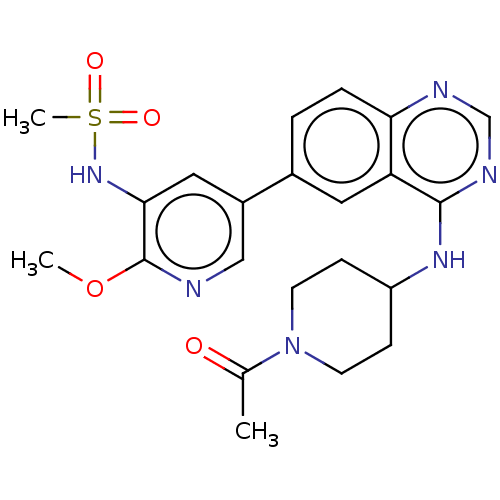
(Homo sapiens (Human)) | BDBM50502319
(CHEMBL4515169)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1ccc2ncnc(NC3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C22H26N6O4S/c1-14(29)28-8-6-17(7-9-28)26-21-18-10-15(4-5-19(18)24-13-25-21)16-11-20(27-33(3,30)31)22(32-2)23-12-16/h4-5,10-13,17,27H,6-9H2,1-3H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K alpha (unknown origin) using lipid substrate measured after 40 mins in presence of ATP by Kinase-Glo plus reagent based luminescen... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.05.043
BindingDB Entry DOI: 10.7270/Q2833W8M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135
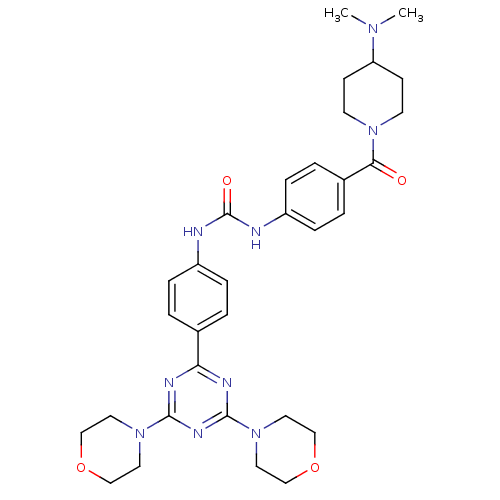
(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of wild type human PI3K p110alpha using PIP2 as substrate by TR-FRET assay |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019
BindingDB Entry DOI: 10.7270/Q2RR22GW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114039
BindingDB Entry DOI: 10.7270/Q2TT4VZR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320095
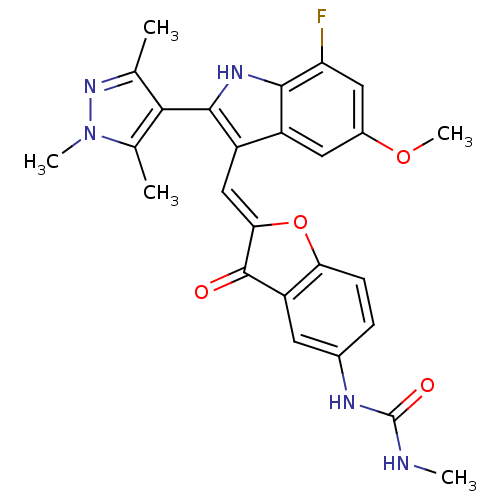
(1-(2-((7-fluoro-5-methoxy-2-(1,3,5-trimethyl-1H-py...)Show SMILES CNC(=O)Nc1ccc2O\C(=C\c3c([nH]c4c(F)cc(OC)cc34)-c3c(C)nn(C)c3C)C(=O)c2c1 |(15.41,-12.32,;16.73,-11.54,;18.07,-12.3,;19.4,-11.53,;18.07,-13.84,;19.41,-14.61,;20.75,-13.83,;22.07,-14.6,;22.07,-16.14,;23.22,-17.16,;22.59,-18.56,;23.36,-19.9,;22.76,-21.32,;23.67,-22.57,;22.77,-23.82,;21.3,-23.34,;19.96,-24.12,;19.97,-25.65,;18.63,-23.35,;18.63,-21.81,;17.29,-21.03,;17.29,-19.49,;19.96,-21.03,;21.3,-21.8,;25.21,-22.57,;26.11,-23.81,;25.63,-25.28,;27.57,-23.34,;27.57,-21.8,;28.82,-20.89,;26.11,-21.32,;25.63,-19.85,;21.06,-18.41,;20.03,-19.56,;20.75,-16.9,;19.42,-16.14,)| Show InChI InChI=1S/C26H24FN5O4/c1-12-22(13(2)32(4)31-12)24-17(16-9-15(35-5)10-19(27)23(16)30-24)11-21-25(33)18-8-14(29-26(34)28-3)6-7-20(18)36-21/h6-11,30H,1-5H3,(H2,28,29,34)/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320103
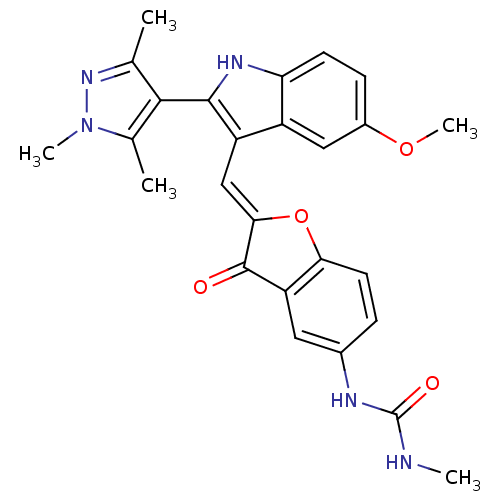
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES CNC(=O)Nc1ccc2O\C(=C\c3c([nH]c4ccc(OC)cc34)-c3c(C)nn(C)c3C)C(=O)c2c1 |(-6.21,5.21,;-4.89,5.98,;-3.55,5.22,;-2.22,5.99,;-3.55,3.68,;-2.21,2.91,;-.87,3.69,;.45,2.92,;.45,1.39,;1.6,.37,;.97,-1.04,;1.74,-2.37,;1.14,-3.79,;2.05,-5.04,;1.14,-6.29,;-.32,-5.82,;-1.66,-6.59,;-2.99,-5.82,;-2.99,-4.28,;-4.33,-3.51,;-4.33,-1.97,;-1.66,-3.51,;-.32,-4.27,;3.59,-5.04,;4.49,-6.29,;4.01,-7.75,;5.95,-5.81,;5.95,-4.27,;7.2,-3.36,;4.49,-3.79,;4.01,-2.33,;-.56,-.88,;-1.59,-2.03,;-.87,.63,;-2.2,1.38,)| Show InChI InChI=1S/C26H25N5O4/c1-13-23(14(2)31(4)30-13)24-18(17-11-16(34-5)7-8-20(17)29-24)12-22-25(32)19-10-15(28-26(33)27-3)6-9-21(19)35-22/h6-12,29H,1-5H3,(H2,27,28,33)/b22-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108124
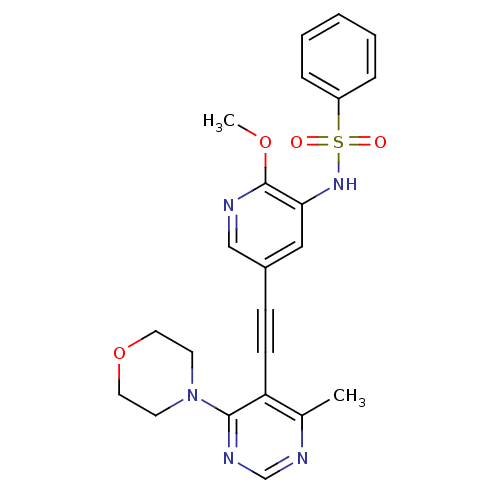
(US8598157, I-1)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccccc1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C23H23N5O4S/c1-17-20(22(26-16-25-17)28-10-12-32-13-11-28)9-8-18-14-21(23(31-2)24-15-18)27-33(29,30)19-6-4-3-5-7-19/h3-7,14-16,27H,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50349621
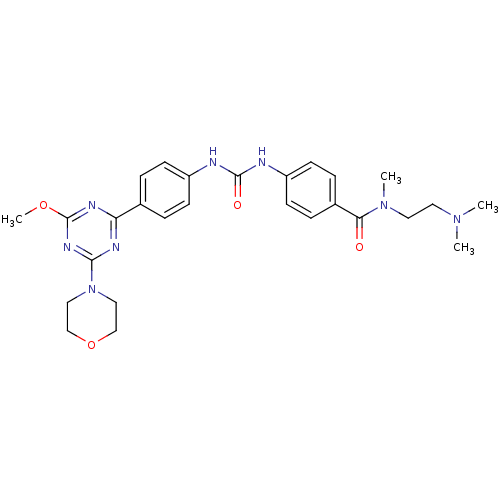
(CHEMBL1808979)Show SMILES COc1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N(C)CCN(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C27H34N8O4/c1-33(2)13-14-34(3)24(36)20-7-11-22(12-8-20)29-26(37)28-21-9-5-19(6-10-21)23-30-25(32-27(31-23)38-4)35-15-17-39-18-16-35/h5-12H,13-18H2,1-4H3,(H2,28,29,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308156
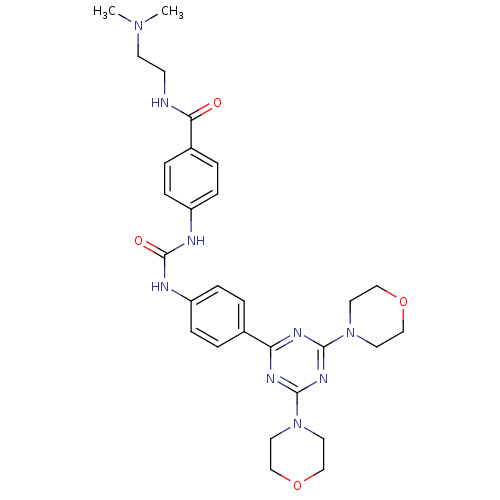
(CHEMBL592615 | N-[2-(Dimethylamino)ethyl]-4-({[4-(...)Show SMILES CN(C)CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C29H37N9O4/c1-36(2)12-11-30-26(39)22-5-9-24(10-6-22)32-29(40)31-23-7-3-21(4-8-23)25-33-27(37-13-17-41-18-14-37)35-28(34-25)38-15-19-42-20-16-38/h3-10H,11-20H2,1-2H3,(H,30,39)(H2,31,32,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50378663

(CHEMBL1204015)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C30H39N9O4/c1-36(2)12-13-37(3)27(40)23-6-10-25(11-7-23)32-30(41)31-24-8-4-22(5-9-24)26-33-28(38-14-18-42-19-15-38)35-29(34-26)39-16-20-43-21-17-39/h4-11H,12-21H2,1-3H3,(H2,31,32,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50388111
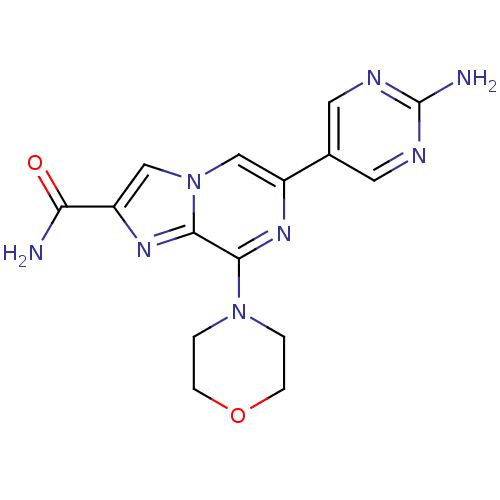
(CHEMBL2058163)Show SMILES NC(=O)c1cn2cc(nc(N3CCOCC3)c2n1)-c1cnc(N)nc1 Show InChI InChI=1S/C15H16N8O2/c16-12(24)11-8-23-7-10(9-5-18-15(17)19-6-9)20-13(14(23)21-11)22-1-3-25-4-2-22/h5-8H,1-4H2,(H2,16,24)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha assessed as accumulation of ADP |
Bioorg Med Chem Lett 22: 5208-14 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.093
BindingDB Entry DOI: 10.7270/Q24Q7W1Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108153

(US8598157, I-30)Show SMILES Cc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C23H22FN5O3S/c1-16-21(23(27-15-26-16)29-9-11-32-12-10-29)8-3-18-13-22(17(2)25-14-18)28-33(30,31)20-6-4-19(24)5-7-20/h4-7,13-15,28H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50579636
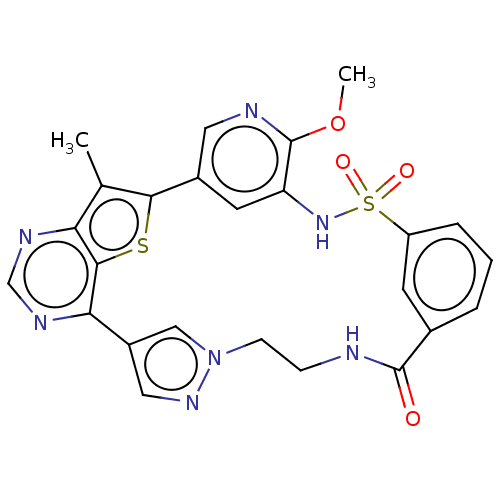
(CHEMBL4876895)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCCn1cc(cn1)-c1ncnc3c(C)c-2sc13 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by ADP-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00412
BindingDB Entry DOI: 10.7270/Q2FX7F91 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108152

(US8598157, I-29)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C23H21F2N5O4S/c1-15-18(22(28-14-27-15)30-7-9-34-10-8-30)5-3-16-11-20(23(33-2)26-13-16)29-35(31,32)21-6-4-17(24)12-19(21)25/h4,6,11-14,29H,7-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108206
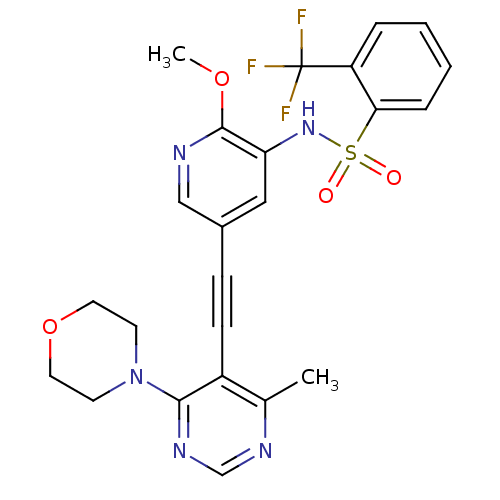
(US8598157, I-83)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccccc1C(F)(F)F)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C24H22F3N5O4S/c1-16-18(22(30-15-29-16)32-9-11-36-12-10-32)8-7-17-13-20(23(35-2)28-14-17)31-37(33,34)21-6-4-3-5-19(21)24(25,26)27/h3-6,13-15,31H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108179
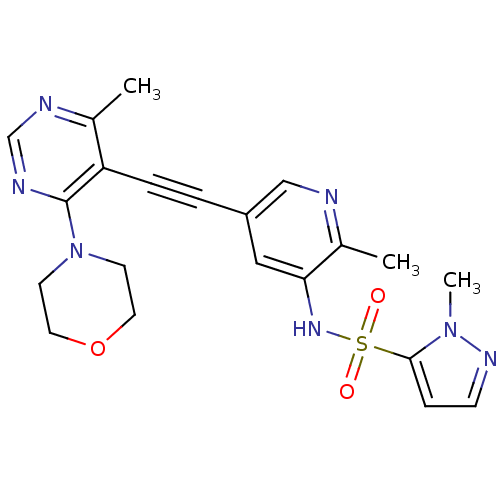
(US8598157, I-56)Show SMILES Cc1ncc(cc1NS(=O)(=O)c1ccnn1C)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C21H23N7O3S/c1-15-18(21(24-14-23-15)28-8-10-31-11-9-28)5-4-17-12-19(16(2)22-13-17)26-32(29,30)20-6-7-25-27(20)3/h6-7,12-14,26H,8-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108210

(US8598157, I-87)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccnn1C)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C21H23N7O4S/c1-15-17(20(24-14-23-15)28-8-10-32-11-9-28)5-4-16-12-18(21(31-3)22-13-16)26-33(29,30)19-6-7-25-27(19)2/h6-7,12-14,26H,8-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
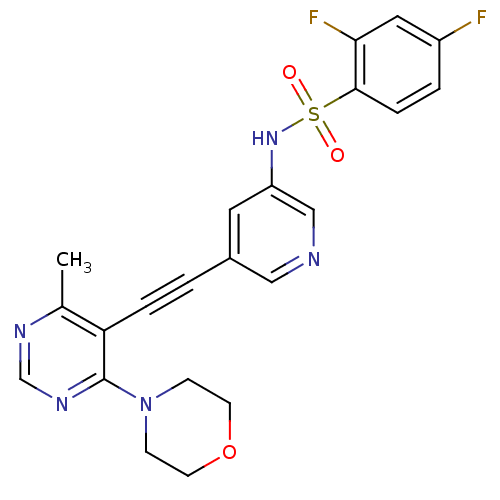
(Homo sapiens (Human)) | BDBM108143
(US8598157, I-20)Show SMILES Cc1ncnc(N2CCOCC2)c1C#Cc1cncc(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C22H19F2N5O3S/c1-15-19(22(27-14-26-15)29-6-8-32-9-7-29)4-2-16-10-18(13-25-12-16)28-33(30,31)21-5-3-17(23)11-20(21)24/h3,5,10-14,28H,6-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
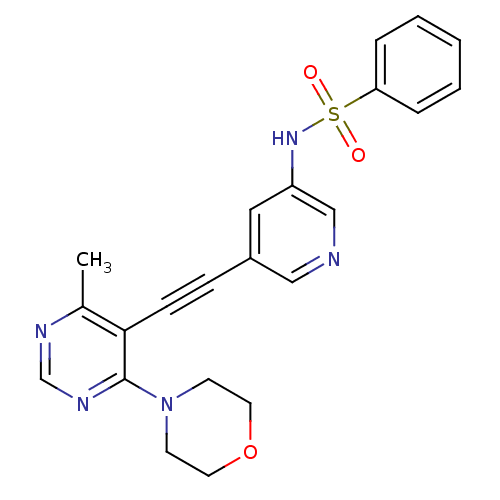
(Homo sapiens (Human)) | BDBM108142
(US8598157, I-19)Show SMILES Cc1ncnc(N2CCOCC2)c1C#Cc1cncc(NS(=O)(=O)c2ccccc2)c1 Show InChI InChI=1S/C22H21N5O3S/c1-17-21(22(25-16-24-17)27-9-11-30-12-10-27)8-7-18-13-19(15-23-14-18)26-31(28,29)20-5-3-2-4-6-20/h2-6,13-16,26H,9-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
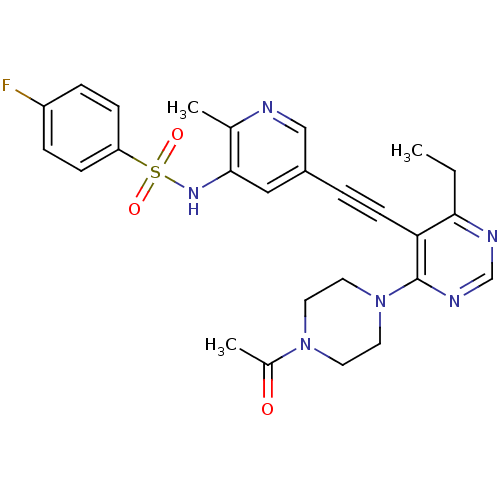
(Homo sapiens (Human)) | BDBM108191
(US8598157, I-68)Show SMILES CCc1ncnc(N2CCN(CC2)C(C)=O)c1C#Cc1cnc(C)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C26H27FN6O3S/c1-4-24-23(26(30-17-29-24)33-13-11-32(12-14-33)19(3)34)10-5-20-15-25(18(2)28-16-20)31-37(35,36)22-8-6-21(27)7-9-22/h6-9,15-17,31H,4,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108175

(US8598157, I-52)Show SMILES Cc1ncc(cc1NS(=O)(=O)c1ccc(Cl)s1)C#Cc1c(C)ncnc1N1CCOCC1 Show InChI InChI=1S/C21H20ClN5O3S2/c1-14-17(21(25-13-24-14)27-7-9-30-10-8-27)4-3-16-11-18(15(2)23-12-16)26-32(28,29)20-6-5-19(22)31-20/h5-6,11-13,26H,7-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM108189

(US8598157, I-66)Show SMILES CCc1ncnc(N2CCC(O)CC2)c1C#Cc1cnc(C)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H26FN5O3S/c1-3-23-22(25(29-16-28-23)31-12-10-20(32)11-13-31)9-4-18-14-24(17(2)27-15-18)30-35(33,34)21-7-5-19(26)6-8-21/h5-8,14-16,20,30,32H,3,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | 7.5 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinas... |
US Patent US8598157 (2013)
BindingDB Entry DOI: 10.7270/Q2X928ZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113428
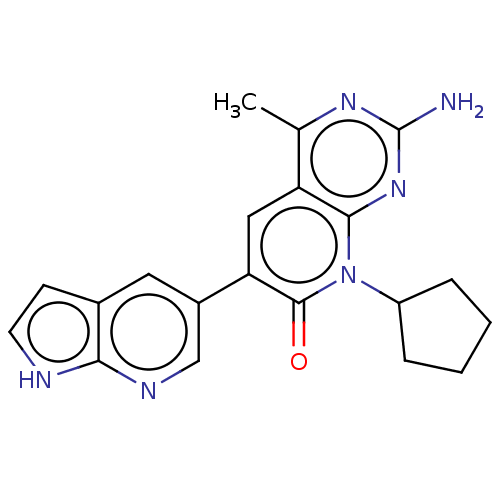
(US8633204, 127)Show SMILES Cc1nc(N)nc2n(C3CCCC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C20H20N6O/c1-11-15-9-16(13-8-12-6-7-22-17(12)23-10-13)19(27)26(14-4-2-3-5-14)18(15)25-20(21)24-11/h6-10,14H,2-5H2,1H3,(H,22,23)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data