 Found 623 hits of ic50 data for polymerid = 1719,50000362
Found 623 hits of ic50 data for polymerid = 1719,50000362 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Chymase
(Homo sapiens (Human)) | BDBM100340
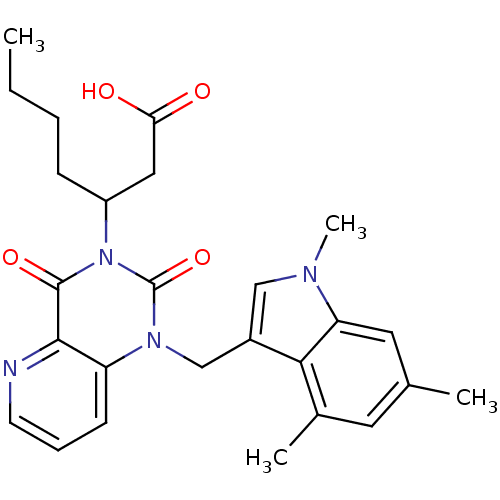
(US8501749, 40)Show SMILES CCCCC(CC(O)=O)n1c(=O)n(Cc2cn(C)c3cc(C)cc(C)c23)c2cccnc2c1=O Show InChI InChI=1S/C26H30N4O4/c1-5-6-8-19(13-22(31)32)30-25(33)24-20(9-7-10-27-24)29(26(30)34)15-18-14-28(4)21-12-16(2)11-17(3)23(18)21/h7,9-12,14,19H,5-6,8,13,15H2,1-4H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100336

(US8501749, 36)Show SMILES CC[C@H](CC(O)=O)n1c(=O)n(Cc2cn(C)c3cc(C)cc(C)c23)c2cccnc2c1=O |r| Show InChI InChI=1S/C24H26N4O4/c1-5-17(11-20(29)30)28-23(31)22-18(7-6-8-25-22)27(24(28)32)13-16-12-26(4)19-10-14(2)9-15(3)21(16)19/h6-10,12,17H,5,11,13H2,1-4H3,(H,29,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50332961
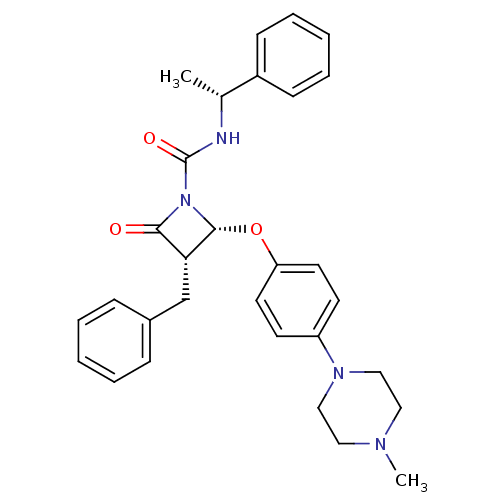
((3S,4S)-3-Benzyl-4-(4-methylpiperazincarbonyl)phen...)Show SMILES C[C@@H](NC(=O)N1[C@H](Oc2ccc(cc2)N2CCN(C)CC2)[C@H](Cc2ccccc2)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C30H34N4O3/c1-22(24-11-7-4-8-12-24)31-30(36)34-28(35)27(21-23-9-5-3-6-10-23)29(34)37-26-15-13-25(14-16-26)33-19-17-32(2)18-20-33/h3-16,22,27,29H,17-21H2,1-2H3,(H,31,36)/t22-,27-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Barkatullah University
Curated by ChEMBL
| Assay Description
Inhibition of human Chymase |
Eur J Med Chem 45: 5541-60 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.035
BindingDB Entry DOI: 10.7270/Q21Z44PQ |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100335

(US8501749, 35)Show SMILES Cc1cc(C)c2c(Cn3c4cccnc4c(=O)n(C(CC(O)=O)C4CC4)c3=O)cn(C)c2c1 Show InChI InChI=1S/C25H26N4O4/c1-14-9-15(2)22-17(12-27(3)20(22)10-14)13-28-18-5-4-8-26-23(18)24(32)29(25(28)33)19(11-21(30)31)16-6-7-16/h4-5,8-10,12,16,19H,6-7,11,13H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100338

(US8501749, 38)Show SMILES CC[C@H](CC(O)=O)n1c(=O)n(Cc2cn(C)c3cc(C)cc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C24H26N4O4/c1-5-17(10-21(29)30)28-23(31)18-11-25-7-6-19(18)27(24(28)32)13-16-12-26(4)20-9-14(2)8-15(3)22(16)20/h6-9,11-12,17H,5,10,13H2,1-4H3,(H,29,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101136

(1,3-Bis-benzo[1,3]dioxol-5-ylmethyl-[1,3]diazetidi...)Show InChI InChI=1S/C18H14N2O6/c21-17-19(7-11-1-3-13-15(5-11)25-9-23-13)18(22)20(17)8-12-2-4-14-16(6-12)26-10-24-14/h1-6H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100324
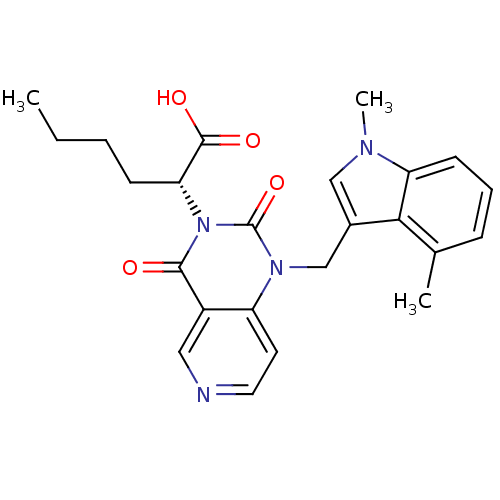
(US8501749, 24)Show SMILES CCCC[C@H](C(O)=O)n1c(=O)n(Cc2cn(C)c3cccc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C24H26N4O4/c1-4-5-8-20(23(30)31)28-22(29)17-12-25-11-10-18(17)27(24(28)32)14-16-13-26(3)19-9-6-7-15(2)21(16)19/h6-7,9-13,20H,4-5,8,14H2,1-3H3,(H,30,31)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100341

(US8501749, 41)Show SMILES CCCC(CC(O)=O)n1c(=O)n(Cc2cn(C)c3cccc(C)c23)c2cccnc2c1=O Show InChI InChI=1S/C24H26N4O4/c1-4-7-17(12-20(29)30)28-23(31)22-19(10-6-11-25-22)27(24(28)32)14-16-13-26(3)18-9-5-8-15(2)21(16)18/h5-6,8-11,13,17H,4,7,12,14H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100339
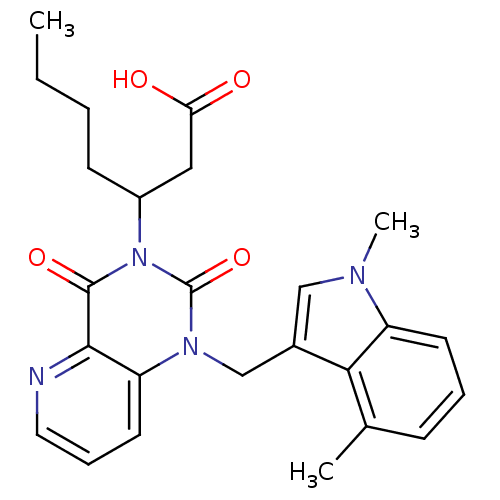
(US8501749, 39)Show SMILES CCCCC(CC(O)=O)n1c(=O)n(Cc2cn(C)c3cccc(C)c23)c2cccnc2c1=O Show InChI InChI=1S/C25H28N4O4/c1-4-5-9-18(13-21(30)31)29-24(32)23-20(11-7-12-26-23)28(25(29)33)15-17-14-27(3)19-10-6-8-16(2)22(17)19/h6-8,10-12,14,18H,4-5,9,13,15H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100330

(US8501749, 30)Show SMILES CC[C@H](CC(O)=O)n1c(=O)n(Cc2cn(C)c3cccc(C)c23)c2cccnc2c1=O |r| Show InChI InChI=1S/C23H24N4O4/c1-4-16(11-19(28)29)27-22(30)21-18(9-6-10-24-21)26(23(27)31)13-15-12-25(3)17-8-5-7-14(2)20(15)17/h5-10,12,16H,4,11,13H2,1-3H3,(H,28,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101135

(1,3-Bis-(4-methoxy-benzyl)-[1,3]diazetidine-2,4-di...)Show InChI InChI=1S/C18H18N2O4/c1-23-15-7-3-13(4-8-15)11-19-17(21)20(18(19)22)12-14-5-9-16(24-2)10-6-14/h3-10H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100320
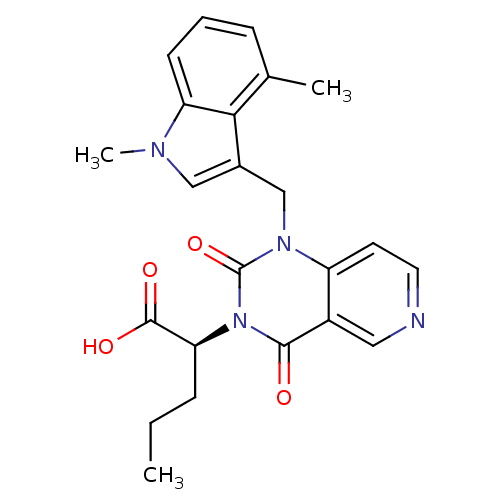
(US8501749, 20)Show SMILES CCC[C@@H](C(O)=O)n1c(=O)n(Cc2cn(C)c3cccc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C23H24N4O4/c1-4-6-19(22(29)30)27-21(28)16-11-24-10-9-17(16)26(23(27)31)13-15-12-25(3)18-8-5-7-14(2)20(15)18/h5,7-12,19H,4,6,13H2,1-3H3,(H,29,30)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100331
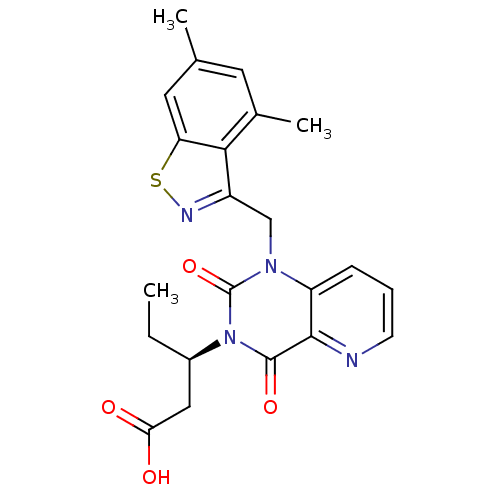
(US8501749, 31)Show SMILES CC[C@H](CC(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2cccnc2c1=O |r| Show InChI InChI=1S/C22H22N4O4S/c1-4-14(10-18(27)28)26-21(29)20-16(6-5-7-23-20)25(22(26)30)11-15-19-13(3)8-12(2)9-17(19)31-24-15/h5-9,14H,4,10-11H2,1-3H3,(H,27,28)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101137
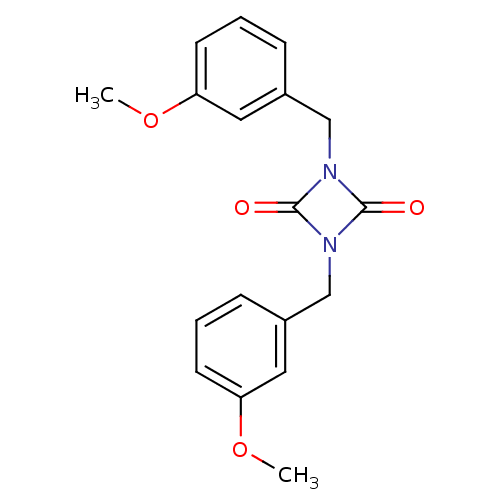
(1,3-Bis-(3-methoxy-benzyl)-[1,3]diazetidine-2,4-di...)Show InChI InChI=1S/C18H18N2O4/c1-23-15-7-3-5-13(9-15)11-19-17(21)20(18(19)22)12-14-6-4-8-16(10-14)24-2/h3-10H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100325
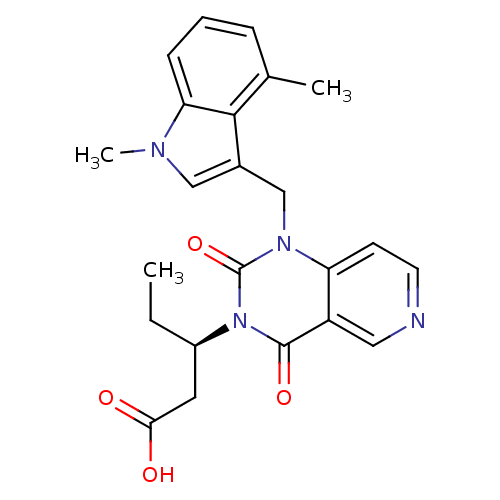
(US8501749, 25)Show SMILES CC[C@H](CC(O)=O)n1c(=O)n(Cc2cn(C)c3cccc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C23H24N4O4/c1-4-16(10-20(28)29)27-22(30)17-11-24-9-8-18(17)26(23(27)31)13-15-12-25(3)19-7-5-6-14(2)21(15)19/h5-9,11-12,16H,4,10,13H2,1-3H3,(H,28,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100334
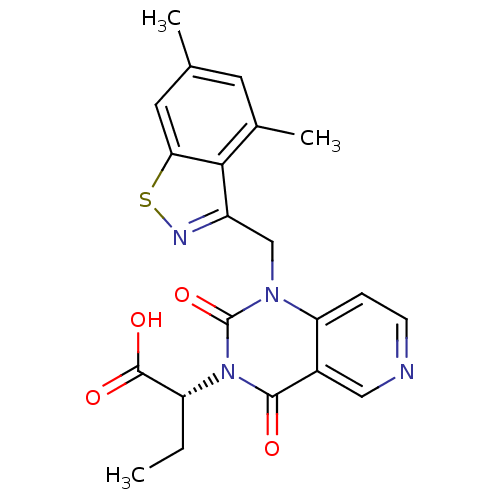
(US8501749, 34)Show SMILES CC[C@H](C(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C21H20N4O4S/c1-4-15(20(27)28)25-19(26)13-9-22-6-5-16(13)24(21(25)29)10-14-18-12(3)7-11(2)8-17(18)30-23-14/h5-9,15H,4,10H2,1-3H3,(H,27,28)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100318
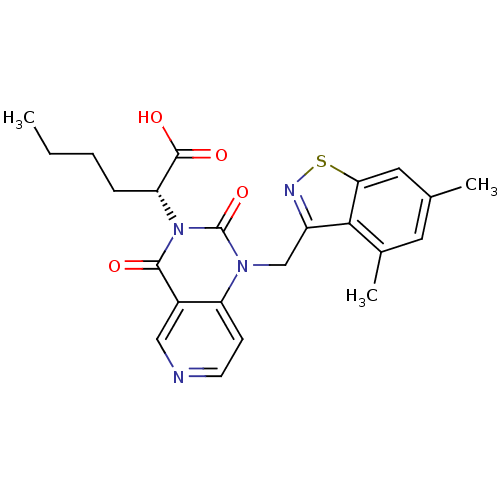
(US8501749, 18)Show SMILES CCCC[C@H](C(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C23H24N4O4S/c1-4-5-6-18(22(29)30)27-21(28)15-11-24-8-7-17(15)26(23(27)31)12-16-20-14(3)9-13(2)10-19(20)32-25-16/h7-11,18H,4-6,12H2,1-3H3,(H,29,30)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100337

(US8501749, 37)Show SMILES CC[C@@H](C(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2cccnc2c1=O |r| Show InChI InChI=1S/C21H20N4O4S/c1-4-14(20(27)28)25-19(26)18-15(6-5-7-22-18)24(21(25)29)10-13-17-12(3)8-11(2)9-16(17)30-23-13/h5-9,14H,4,10H2,1-3H3,(H,27,28)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM134267
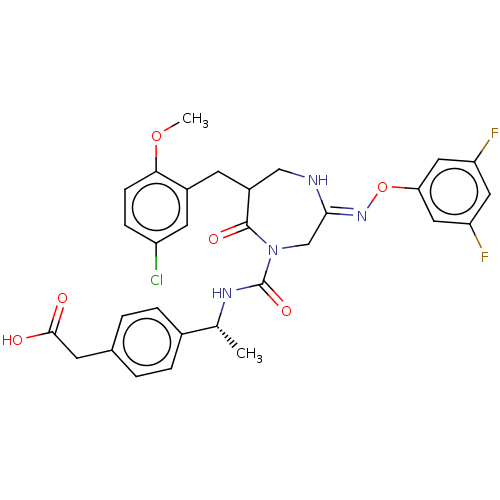
(US8846660, 41)Show SMILES COc1ccc(Cl)cc1CC1CN\C(CN(C(=O)N[C@H](C)c2ccc(CC(O)=O)cc2)C1=O)=N/Oc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29ClF2N4O6/c1-17(19-5-3-18(4-6-19)9-28(38)39)35-30(41)37-16-27(36-43-25-13-23(32)12-24(33)14-25)34-15-21(29(37)40)10-20-11-22(31)7-8-26(20)42-2/h3-8,11-14,17,21H,9-10,15-16H2,1-2H3,(H,34,36)(H,35,41)(H,38,39)/t17-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd.
US Patent
| Assay Description
The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... |
US Patent US8846660 (2014)
BindingDB Entry DOI: 10.7270/Q22V2DTB |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100327
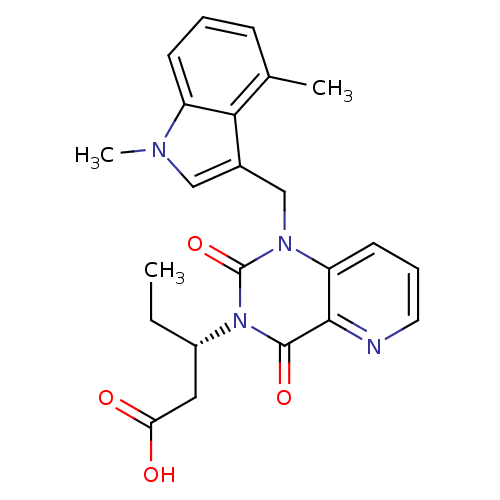
(US8501749, 27)Show SMILES CC[C@@H](CC(O)=O)n1c(=O)n(Cc2cn(C)c3cccc(C)c23)c2cccnc2c1=O |r| Show InChI InChI=1S/C23H24N4O4/c1-4-16(11-19(28)29)27-22(30)21-18(9-6-10-24-21)26(23(27)31)13-15-12-25(3)17-8-5-7-14(2)20(15)17/h5-10,12,16H,4,11,13H2,1-3H3,(H,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100312

(US8501749, 12)Show SMILES Cc1cccc2n(C)cc(Cn3c4ccncc4c(=O)n([C@H](C(O)=O)c4ccccc4)c3=O)c12 |r| Show InChI InChI=1S/C26H22N4O4/c1-16-7-6-10-21-22(16)18(14-28(21)2)15-29-20-11-12-27-13-19(20)24(31)30(26(29)34)23(25(32)33)17-8-4-3-5-9-17/h3-14,23H,15H2,1-2H3,(H,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50332960
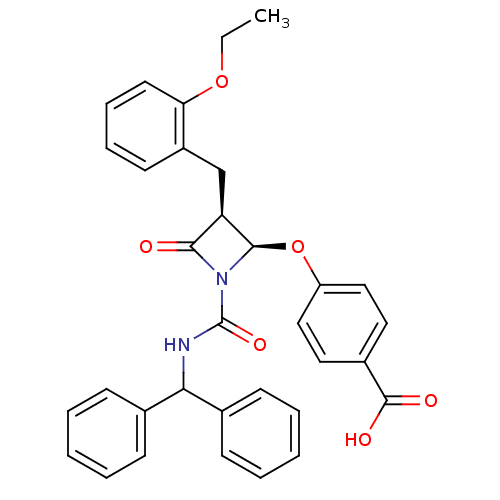
((3S,4S)-4-(4-Carboxy)phenoxy-1-[diphenylmethyl)ami...)Show SMILES CCOc1ccccc1C[C@H]1[C@@H](Oc2ccc(cc2)C(O)=O)N(C(=O)NC(c2ccccc2)c2ccccc2)C1=O |r| Show InChI InChI=1S/C33H30N2O6/c1-2-40-28-16-10-9-15-25(28)21-27-30(36)35(31(27)41-26-19-17-24(18-20-26)32(37)38)33(39)34-29(22-11-5-3-6-12-22)23-13-7-4-8-14-23/h3-20,27,29,31H,2,21H2,1H3,(H,34,39)(H,37,38)/t27-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Barkatullah University
Curated by ChEMBL
| Assay Description
Inhibition of human Chymase |
Eur J Med Chem 45: 5541-60 (2010)
Article DOI: 10.1016/j.ejmech.2010.09.035
BindingDB Entry DOI: 10.7270/Q21Z44PQ |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208225
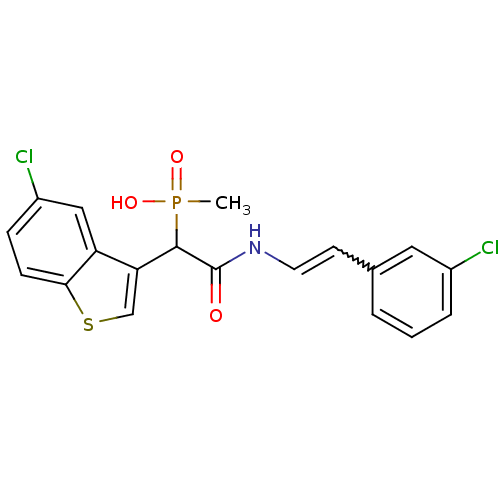
((E)-2-(3-chlorostyrylamino)-1-(5-chlorobenzo[b]thi...)Show SMILES CP(O)(=O)C(C(=O)NC=Cc1cccc(Cl)c1)c1csc2ccc(Cl)cc12 |w:9.9| Show InChI InChI=1S/C19H16Cl2NO3PS/c1-26(24,25)18(16-11-27-17-6-5-14(21)10-15(16)17)19(23)22-8-7-12-3-2-4-13(20)9-12/h2-11,18H,1H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50101132

(1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...)Show InChI InChI=1S/C16H14N2O2/c19-15-17(11-13-7-3-1-4-8-13)16(20)18(15)12-14-9-5-2-6-10-14/h1-10H,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against human Serine protease chymase |
Bioorg Med Chem Lett 11: 1691-4 (2001)
BindingDB Entry DOI: 10.7270/Q2V12421 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100321

(US8501749, 21)Show SMILES CCC[C@H](C(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C22H22N4O4S/c1-4-5-17(21(28)29)26-20(27)14-10-23-7-6-16(14)25(22(26)30)11-15-19-13(3)8-12(2)9-18(19)31-24-15/h6-10,17H,4-5,11H2,1-3H3,(H,28,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM134262
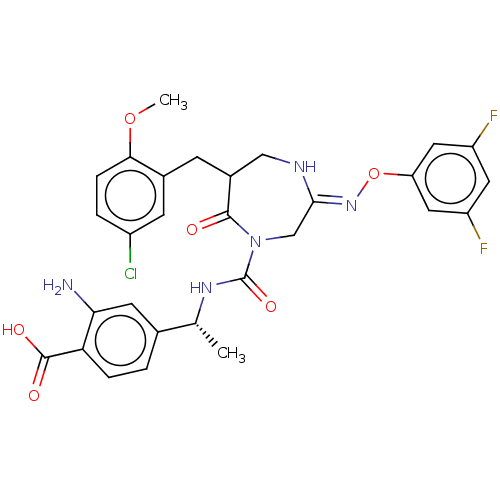
(US8846660, 30)Show SMILES COc1ccc(Cl)cc1CC1CN\C(CN(C(=O)N[C@H](C)c2ccc(C(O)=O)c(N)c2)C1=O)=N/Oc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C29H28ClF2N5O6/c1-15(16-3-5-23(28(39)40)24(33)9-16)35-29(41)37-14-26(36-43-22-11-20(31)10-21(32)12-22)34-13-18(27(37)38)7-17-8-19(30)4-6-25(17)42-2/h3-6,8-12,15,18H,7,13-14,33H2,1-2H3,(H,34,36)(H,35,41)(H,39,40)/t15-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd.
US Patent
| Assay Description
The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... |
US Patent US8846660 (2014)
BindingDB Entry DOI: 10.7270/Q22V2DTB |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208224
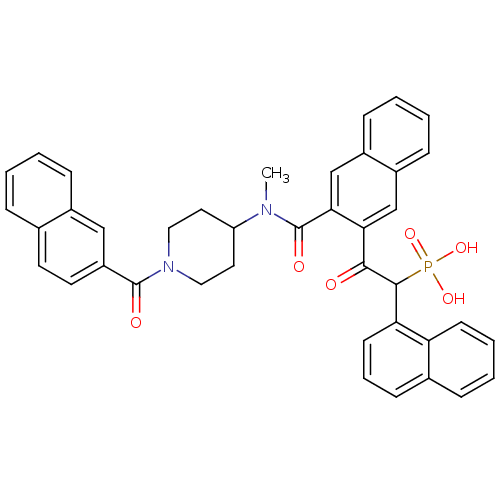
(2-(3-((1-(2-naphthoyl)piperidin-4-yl)(methyl)carba...)Show SMILES CN(C1CCN(CC1)C(=O)c1ccc2ccccc2c1)C(=O)c1cc2ccccc2cc1C(=O)C(c1cccc2ccccc12)P(O)(O)=O Show InChI InChI=1S/C40H35N2O6P/c1-41(32-19-21-42(22-20-32)39(44)31-18-17-26-9-2-3-11-28(26)23-31)40(45)36-25-30-13-5-4-12-29(30)24-35(36)37(43)38(49(46,47)48)34-16-8-14-27-10-6-7-15-33(27)34/h2-18,23-25,32,38H,19-22H2,1H3,(H2,46,47,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymase
(Homo sapiens (Human)) | BDBM100322
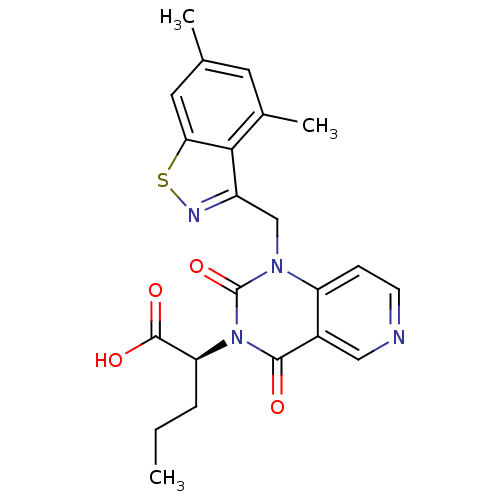
(US8501749, 22)Show SMILES CCC[C@@H](C(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C22H22N4O4S/c1-4-5-17(21(28)29)26-20(27)14-10-23-7-6-16(14)25(22(26)30)11-15-19-13(3)8-12(2)9-18(19)31-24-15/h6-10,17H,4-5,11H2,1-3H3,(H,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50075109
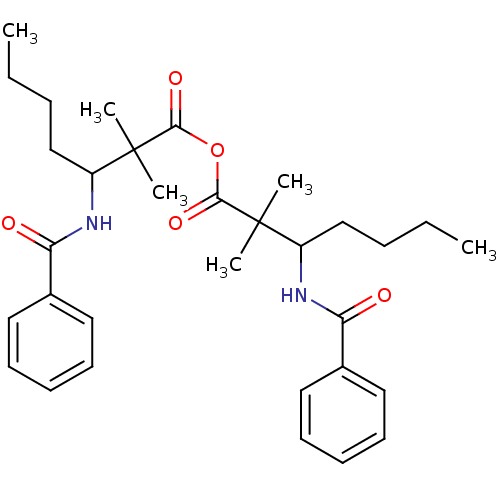
(1,1-dimethyl-2-phenylcarboxamidoheptanoic anhydrid...)Show SMILES CCCCC(NC(=O)c1ccccc1)C(C)(C)C(=O)OC(=O)C(C)(C)C(CCCC)NC(=O)c1ccccc1 Show InChI InChI=1S/C32H44N2O5/c1-7-9-21-25(33-27(35)23-17-13-11-14-18-23)31(3,4)29(37)39-30(38)32(5,6)26(22-10-8-2)34-28(36)24-19-15-12-16-20-24/h11-20,25-26H,7-10,21-22H2,1-6H3,(H,33,35)(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human chymase (h-chymase) |
Bioorg Med Chem Lett 9: 413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2BK1BGC |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50075107

(1,1-dimethyl-4-phenyl-2-propylcarboxamidopentanoic...)Show SMILES CCCC(=O)NC(CCc1ccccc1)C(C)(C)C(=O)OC(=O)C(C)(C)C(CCc1ccccc1)NC(=O)CCC Show InChI InChI=1S/C34H48N2O5/c1-7-15-29(37)35-27(23-21-25-17-11-9-12-18-25)33(3,4)31(39)41-32(40)34(5,6)28(36-30(38)16-8-2)24-22-26-19-13-10-14-20-26/h9-14,17-20,27-28H,7-8,15-16,21-24H2,1-6H3,(H,35,37)(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine pancreatic alpha-chymotrypsin (alpha-CT) |
Bioorg Med Chem Lett 9: 413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2BK1BGC |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50075107

(1,1-dimethyl-4-phenyl-2-propylcarboxamidopentanoic...)Show SMILES CCCC(=O)NC(CCc1ccccc1)C(C)(C)C(=O)OC(=O)C(C)(C)C(CCc1ccccc1)NC(=O)CCC Show InChI InChI=1S/C34H48N2O5/c1-7-15-29(37)35-27(23-21-25-17-11-9-12-18-25)33(3,4)31(39)41-32(40)34(5,6)28(36-30(38)16-8-2)24-22-26-19-13-10-14-20-26/h9-14,17-20,27-28H,7-8,15-16,21-24H2,1-6H3,(H,35,37)(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human chymase (h-chymase) |
Bioorg Med Chem Lett 9: 413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2BK1BGC |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50093750

((6R,7R)-3-(1-Carboxymethyl-1H-tetrazol-5-ylsulfany...)Show SMILES COc1ccccc1C(=O)N[C@@]1(OC)[C@H]2OCC(CSc3nnnn3CC(O)=O)=C(N2C1=O)C(=O)OCc1cccc(C)c1 |c:30| Show InChI InChI=1S/C28H28N6O9S/c1-16-7-6-8-17(11-16)13-42-24(38)22-18(15-44-27-30-31-32-33(27)12-21(35)36)14-43-26-28(41-3,25(39)34(22)26)29-23(37)19-9-4-5-10-20(19)40-2/h4-11,26H,12-15H2,1-3H3,(H,29,37)(H,35,36)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Serine protease chymase |
Bioorg Med Chem Lett 10: 2403-6 (2001)
BindingDB Entry DOI: 10.7270/Q23R0S4F |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50093722

((6R,7R)-3-(4-Carboxymethyl-4H-[1,2,4]triazol-3-yls...)Show SMILES COc1ccccc1C(=O)N[C@@]1(OC)[C@H]2OCC(CSc3nncn3CC(O)=O)=C(N2C1=O)C(=O)OCc1cccc(C)c1 |c:30| Show InChI InChI=1S/C29H29N5O9S/c1-17-7-6-8-18(11-17)13-42-25(38)23-19(15-44-28-32-30-16-33(28)12-22(35)36)14-43-27-29(41-3,26(39)34(23)27)31-24(37)20-9-4-5-10-21(20)40-2/h4-11,16,27H,12-15H2,1-3H3,(H,31,37)(H,35,36)/t27-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against human serine protease chymase |
Bioorg Med Chem Lett 10: 2397-401 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HVR |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100308

(US8501749, 8)Show SMILES Cc1cccc2n(C)cc(Cn3c4cccnc4c(=O)n(CC(O)=O)c3=O)c12 Show InChI InChI=1S/C20H18N4O4/c1-12-5-3-6-14-17(12)13(9-22(14)2)10-23-15-7-4-8-21-18(15)19(27)24(20(23)28)11-16(25)26/h3-9H,10-11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100326

(US8501749, 26)Show SMILES CC[C@H](CC(O)=O)n1c(=O)n(Cc2cn(C)c3cccc(C)c23)c2cnccc2c1=O |r| Show InChI InChI=1S/C23H24N4O4/c1-4-16(10-20(28)29)27-22(30)17-8-9-24-11-19(17)26(23(27)31)13-15-12-25(3)18-7-5-6-14(2)21(15)18/h5-9,11-12,16H,4,10,13H2,1-3H3,(H,28,29)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM144347

(US8969348, {4-[(1,4-Dimethyl-1H-indol-3-yl)methyl]...)Show SMILES Cc1cccc2n(C)cc(Cc3nn(C(C(O)=O)c4ccccc4)c(=O)c4ccccc34)c12 Show InChI InChI=1S/C27H23N3O3/c1-17-9-8-14-23-24(17)19(16-29(23)2)15-22-20-12-6-7-13-21(20)26(31)30(28-22)25(27(32)33)18-10-4-3-5-11-18/h3-14,16,25H,15H2,1-2H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
Chymase assays were performed in a total volume of 15 uL in Corning black opaque 384-well microtiter plates with a non-binding surface (Corning, N.Y.... |
US Patent US8969348 (2015)
BindingDB Entry DOI: 10.7270/Q2J38R8B |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100319
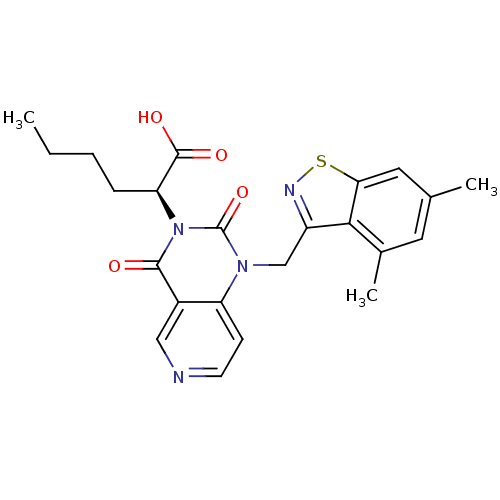
(US8501749, 19)Show SMILES CCCC[C@@H](C(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C23H24N4O4S/c1-4-5-6-18(22(29)30)27-21(28)15-11-24-8-7-17(15)26(23(27)31)12-16-20-14(3)9-13(2)10-19(20)32-25-16/h7-11,18H,4-6,12H2,1-3H3,(H,29,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100317

(US8501749, 17)Show SMILES CC[C@@H](C(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C21H20N4O4S/c1-4-15(20(27)28)25-19(26)13-9-22-6-5-16(13)24(21(25)29)10-14-18-12(3)7-11(2)8-17(18)30-23-14/h5-9,15H,4,10H2,1-3H3,(H,27,28)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM134278
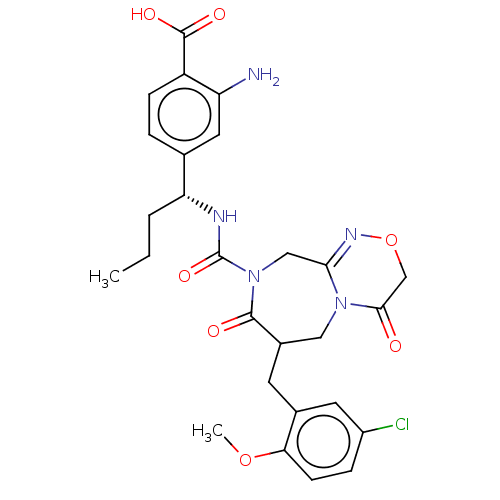
(US8846660, 96)Show SMILES CCC[C@@H](NC(=O)N1CC2=NOCC(=O)N2CC(Cc2cc(Cl)ccc2OC)C1=O)c1ccc(C(O)=O)c(N)c1 |r,t:9| Show InChI InChI=1S/C27H30ClN5O7/c1-3-4-21(15-5-7-19(26(36)37)20(29)11-15)30-27(38)33-13-23-31-40-14-24(34)32(23)12-17(25(33)35)9-16-10-18(28)6-8-22(16)39-2/h5-8,10-11,17,21H,3-4,9,12-14,29H2,1-2H3,(H,30,38)(H,36,37)/t17?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd.
US Patent
| Assay Description
The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... |
US Patent US8846660 (2014)
BindingDB Entry DOI: 10.7270/Q22V2DTB |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100333
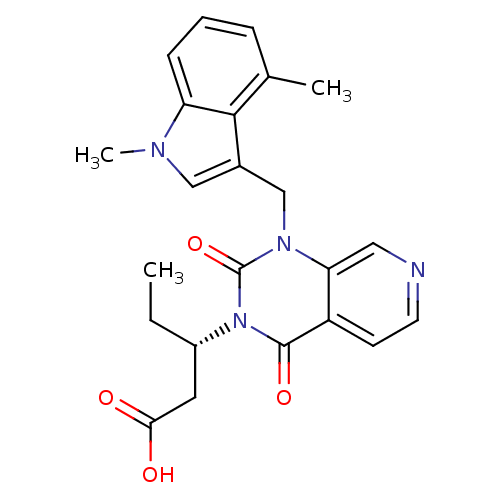
(US8501749, 33)Show SMILES CC[C@@H](CC(O)=O)n1c(=O)n(Cc2cn(C)c3cccc(C)c23)c2cnccc2c1=O |r| Show InChI InChI=1S/C23H24N4O4/c1-4-16(10-20(28)29)27-22(30)17-8-9-24-11-19(17)26(23(27)31)13-15-12-25(3)18-7-5-6-14(2)21(15)18/h5-9,11-12,16H,4,10,13H2,1-3H3,(H,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100315

(US8501749, 15)Show SMILES CC[C@@H](CC(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2cccnc2c1=O |r| Show InChI InChI=1S/C22H22N4O4S/c1-4-14(10-18(27)28)26-21(29)20-16(6-5-7-23-20)25(22(26)30)11-15-19-13(3)8-12(2)9-17(19)31-24-15/h5-9,14H,4,10-11H2,1-3H3,(H,27,28)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50452609

(CHEMBL4206099)Show SMILES CC[C@@H](NC(=O)N1C\C(NC[C@@H](Cc2cc(Cl)ccc2OC)C1=O)=N\Oc1ccccc1)c1ccc(C(O)=O)c(N)c1 |r| Show InChI InChI=1S/C30H32ClN5O6/c1-3-25(18-9-11-23(29(38)39)24(32)15-18)34-30(40)36-17-27(35-42-22-7-5-4-6-8-22)33-16-20(28(36)37)13-19-14-21(31)10-12-26(19)41-2/h4-12,14-15,20,25H,3,13,16-17,32H2,1-2H3,(H,33,35)(H,34,40)(H,38,39)/t20-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu... |
Bioorg Med Chem Lett 28: 188-192 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.031
BindingDB Entry DOI: 10.7270/Q2T72M14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymase
(Homo sapiens (Human)) | BDBM144346
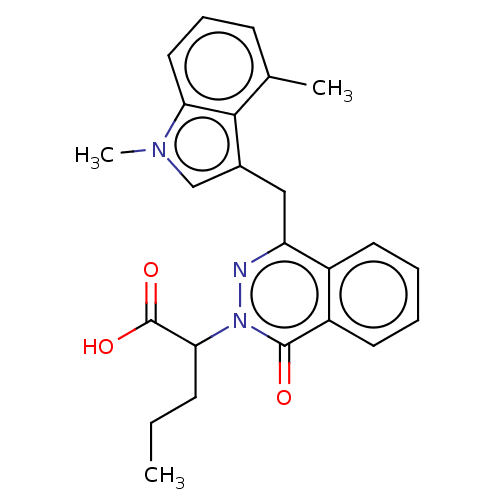
(US8969348, 2-{4-[(1,4-Dimethyl-1H-indol-3-yl)methy...)Show SMILES CCCC(C(O)=O)n1nc(Cc2cn(C)c3cccc(C)c23)c2ccccc2c1=O Show InChI InChI=1S/C24H25N3O3/c1-4-8-21(24(29)30)27-23(28)18-11-6-5-10-17(18)19(25-27)13-16-14-26(3)20-12-7-9-15(2)22(16)20/h5-7,9-12,14,21H,4,8,13H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
Chymase assays were performed in a total volume of 15 uL in Corning black opaque 384-well microtiter plates with a non-binding surface (Corning, N.Y.... |
US Patent US8969348 (2015)
BindingDB Entry DOI: 10.7270/Q2J38R8B |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM134276

(US8846660, 76)Show SMILES CC[C@@H](NC(=O)N1C\C(NCC(Cc2cc(Cl)ccc2OC)C1=O)=N\Oc1ccccc1)c1ccc(C(O)=O)c(N)c1 |r| Show InChI InChI=1S/C30H32ClN5O6/c1-3-25(18-9-11-23(29(38)39)24(32)15-18)34-30(40)36-17-27(35-42-22-7-5-4-6-8-22)33-16-20(28(36)37)13-19-14-21(31)10-12-26(19)41-2/h4-12,14-15,20,25H,3,13,16-17,32H2,1-2H3,(H,33,35)(H,34,40)(H,38,39)/t20?,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd.
US Patent
| Assay Description
The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... |
US Patent US8846660 (2014)
BindingDB Entry DOI: 10.7270/Q22V2DTB |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50288847
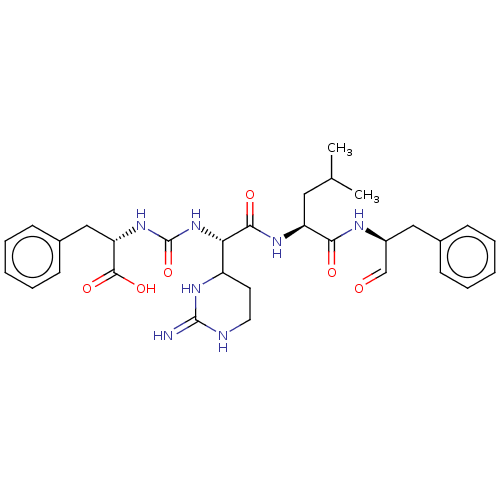
(Chymostatin)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C1CCNC(=N)N1)C(=O)N[C@@H](Cc1ccccc1)C=O |r| Show InChI InChI=1S/C9H13N3O6/c13-6(3-12-5-10-4-11-12)1-2-18-7(8(14)15)9(16)17/h4-7,13H,1-3H2,(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of chymase (unknown origin) by fluorescence assay |
J Med Chem 61: 6364-6378 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00885
BindingDB Entry DOI: 10.7270/Q2N87D9X |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100307
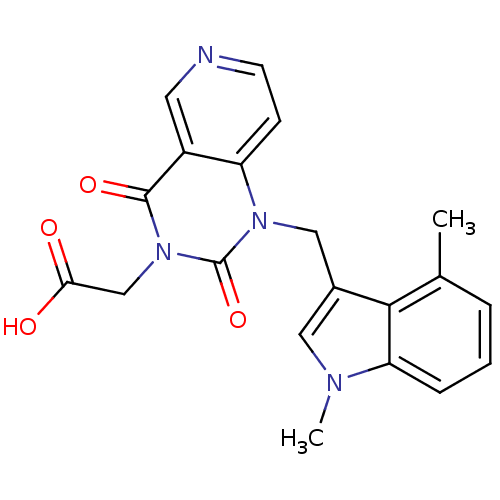
(US8501749, 7)Show SMILES Cc1cccc2n(C)cc(Cn3c4ccncc4c(=O)n(CC(O)=O)c3=O)c12 Show InChI InChI=1S/C20H18N4O4/c1-12-4-3-5-16-18(12)13(9-22(16)2)10-23-15-6-7-21-8-14(15)19(27)24(20(23)28)11-17(25)26/h3-9H,10-11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM100329

(US8501749, 29)Show SMILES CC[C@H](CC(O)=O)n1c(=O)n(Cc2nsc3cc(C)cc(C)c23)c2ccncc2c1=O |r| Show InChI InChI=1S/C22H22N4O4S/c1-4-14(9-19(27)28)26-21(29)15-10-23-6-5-17(15)25(22(26)30)11-16-20-13(3)7-12(2)8-18(20)31-24-16/h5-8,10,14H,4,9,11H2,1-3H3,(H,27,28)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
In vitro inhibition assay of Chymase. |
US Patent US8501749 (2013)
BindingDB Entry DOI: 10.7270/Q28G8JB2 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM134266

(US8846660, 40)Show SMILES COc1ccc(Cl)cc1CC1CN\C(CN(C(=O)N[C@H](C)c2ccc(CC(O)=O)cc2)C1=O)=N/Oc1ccc(F)cc1 |r| Show InChI InChI=1S/C30H30ClFN4O6/c1-18(20-5-3-19(4-6-20)13-28(37)38)34-30(40)36-17-27(35-42-25-10-8-24(32)9-11-25)33-16-22(29(36)39)14-21-15-23(31)7-12-26(21)41-2/h3-12,15,18,22H,13-14,16-17H2,1-2H3,(H,33,35)(H,34,40)(H,37,38)/t18-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd.
US Patent
| Assay Description
The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... |
US Patent US8846660 (2014)
BindingDB Entry DOI: 10.7270/Q22V2DTB |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM337803

(2,4-Dioxo-1-[4-(2-oxo-1,3-oxazolidin-3-yl)phenyl]-...)Show SMILES OC(=O)c1cn(-c2ccc(cc2)N2CCOC2=O)c(=O)n(C2CCCc3c2cccc3C(F)(F)F)c1=O Show InChI InChI=1S/C25H20F3N3O6/c26-25(27,28)19-5-1-4-17-16(19)3-2-6-20(17)31-21(32)18(22(33)34)13-30(23(31)35)15-9-7-14(8-10-15)29-11-12-37-24(29)36/h1,4-5,7-10,13,20H,2-3,6,11-12H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft
US Patent
| Assay Description
The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used fo... |
US Patent US9751843 (2017)
BindingDB Entry DOI: 10.7270/Q2V98B6B |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50208236
(1-(5-chloro-1-methyl-1H-indol-3-yl)-2-(naphthalen-...)Show SMILES Cn1cc(C(C(=O)Nc2ccc3ccccc3c2)P(C)(O)=O)c2cc(Cl)ccc12 Show InChI InChI=1S/C22H20ClN2O3P/c1-25-13-19(18-12-16(23)8-10-20(18)25)21(29(2,27)28)22(26)24-17-9-7-14-5-3-4-6-15(14)11-17/h3-13,21H,1-2H3,(H,24,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human skin chymase |
J Med Chem 50: 1727-30 (2007)
Article DOI: 10.1021/jm0700619
BindingDB Entry DOI: 10.7270/Q26W99RF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data