

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymase (Homo sapiens (Human)) | BDBM50520051 (CHEMBL4592765) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098853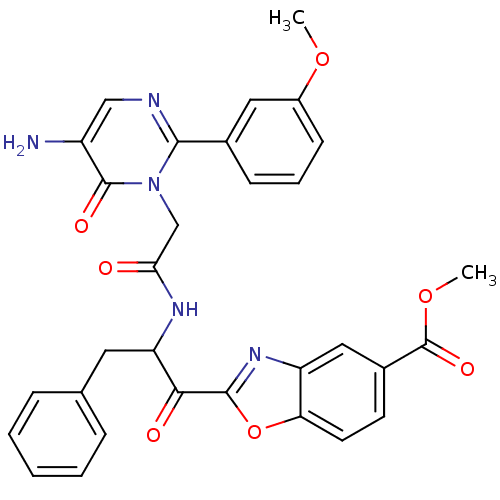 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against canine skin chymase | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068901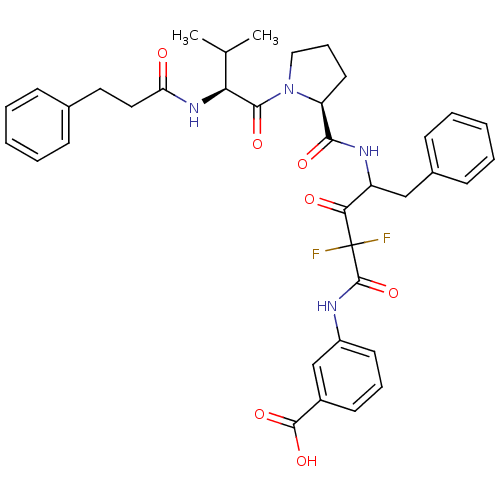 (3-[2,2-Difluoro-4-({(S)-1-[(S)-3-methyl-2-(3-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50520048 (CHEMBL4450993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50520049 (CHEMBL4560112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50520060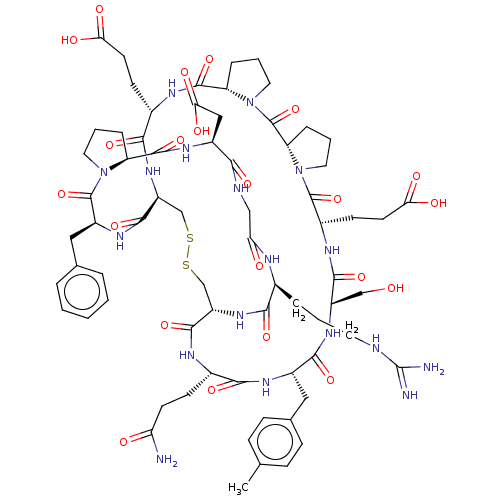 (CHEMBL4465306) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208224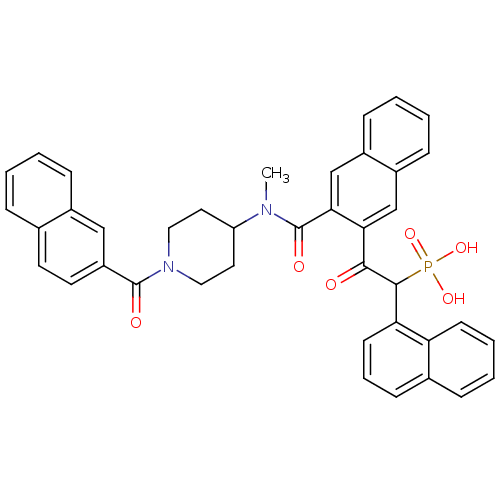 (2-(3-((1-(2-naphthoyl)piperidin-4-yl)(methyl)carba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50139758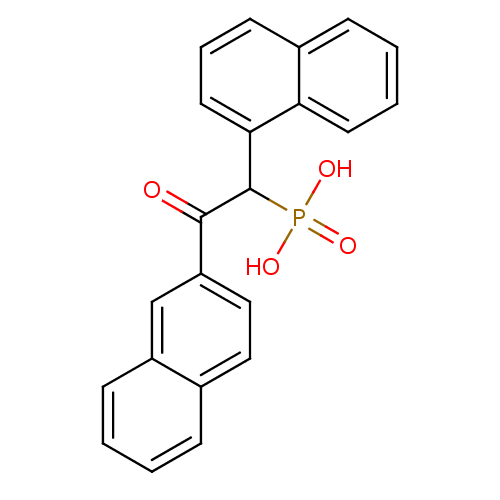 ((2-Naphthalen-2-yl-1-naphthalen-1-yl-2-oxo-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of chymase in human mast cells using Suc-Ala-Ala-Pro-Phe-(p-nitroanilide) as substrate for 15 mins by spectrophotometric method | Eur J Med Chem 161: 252-276 (2019) Article DOI: 10.1016/j.ejmech.2018.10.018 BindingDB Entry DOI: 10.7270/Q25M690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098874 (4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068918 ((S)-4-((2S,3S)-2-Benzyloxycarbonylamino-3-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068899 (3-(4-{[(S)-1-((S)-2-Benzoylamino-3-methyl-butyryl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098868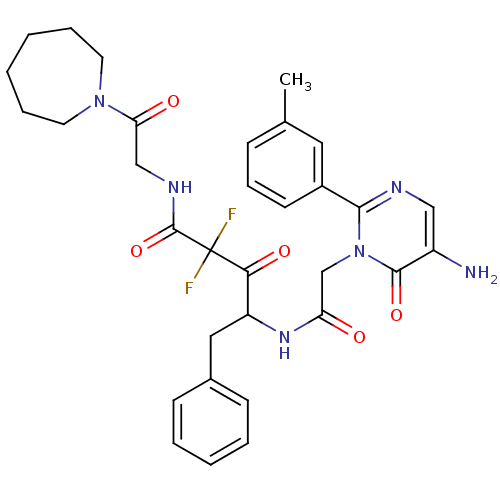 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098847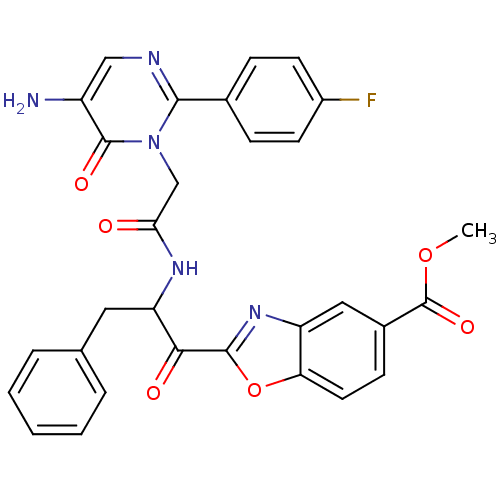 (2-(2-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against canine skin chymase | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068919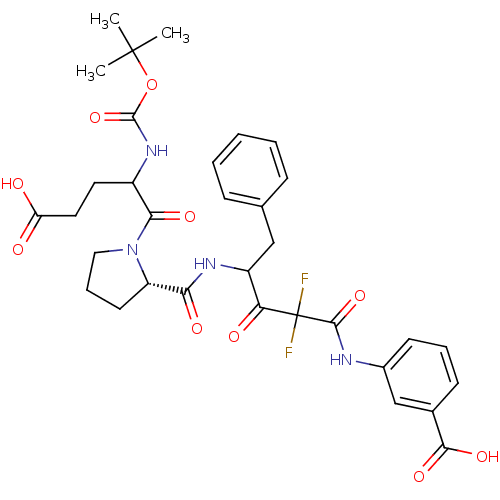 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-4-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50374400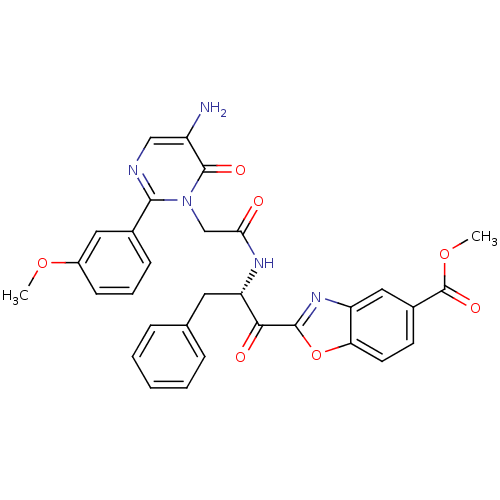 (CHEMBL402185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human chymase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098853 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity evaluated against chymase from human heart. | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098854 (2-{2-[2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity evaluated against chymase from human heart. | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098841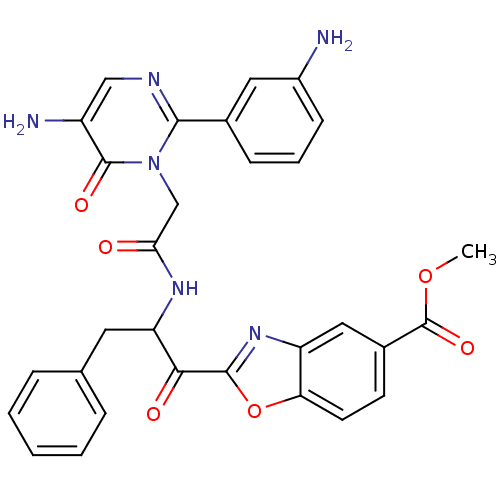 (2-(2-{2-[5-Amino-2-(3-amino-phenyl)-6-oxo-6H-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity evaluated against chymase from human heart. | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50374284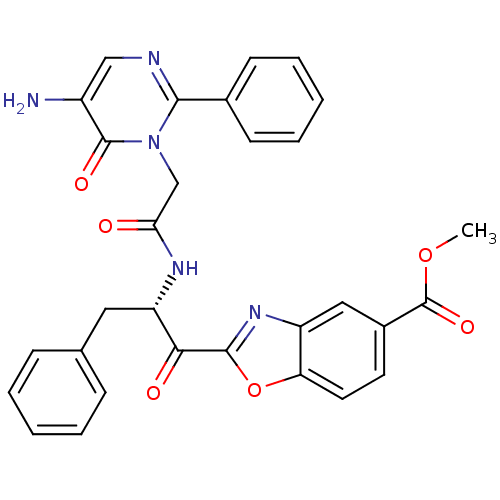 (CHEMBL256270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human chymase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068894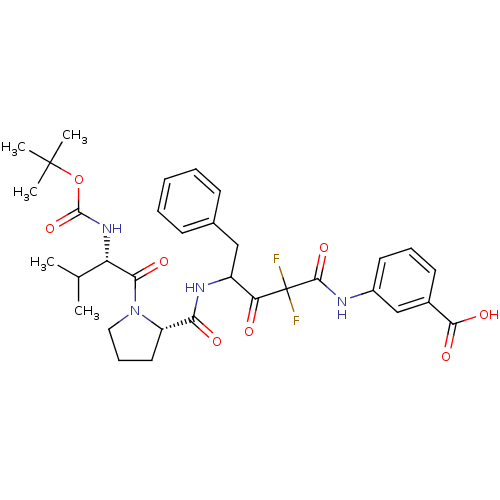 (3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068911 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068894 (3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50520063 (CHEMBL4586444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068917 (5-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-4-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068889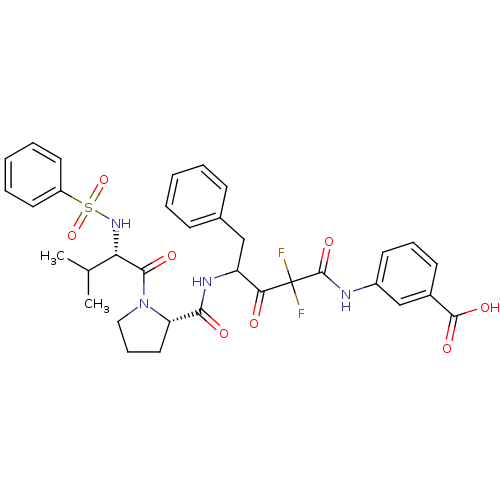 (3-(4-{[(S)-1-((S)-2-Benzenesulfonylamino-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068896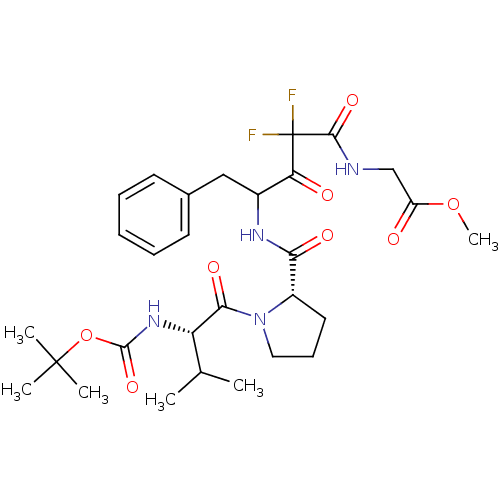 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50520050 (CHEMBL4457132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068915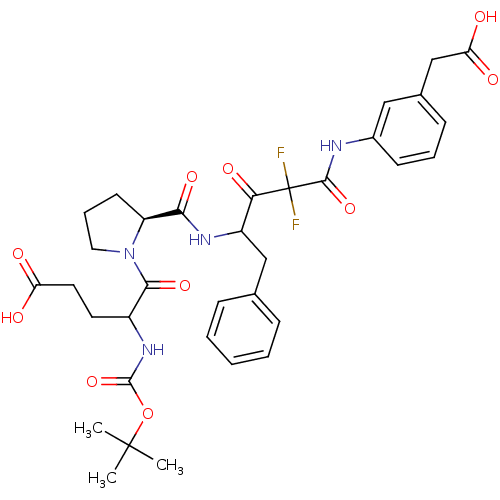 (5-{(S)-2-[1-Benzyl-3-(3-carboxymethyl-phenylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068887 (4-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068892 (3-(4-{[(S)-1-((S)-2-Acetylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50520064 (CHEMBL4590739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098892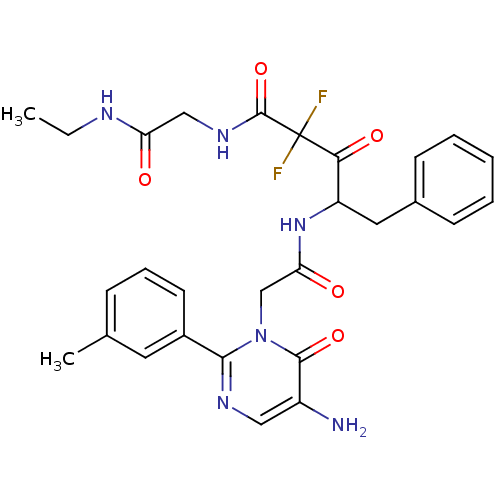 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098879 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098880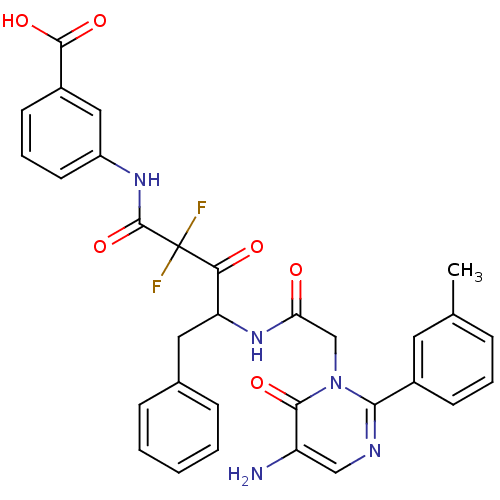 (3-{4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50520066 (CHEMBL4442550) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098870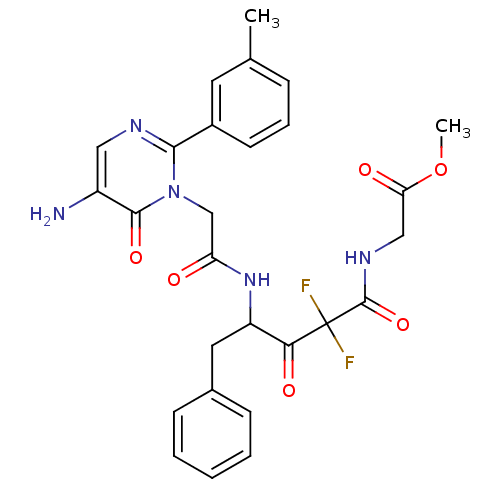 (CHEMBL26182 | {4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098878 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208222 ((E)-2-(3-chloro-5-fluorostyrylamino)-1-(5-chlorobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50520059 (CHEMBL4561218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098875 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068903 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068905 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM87059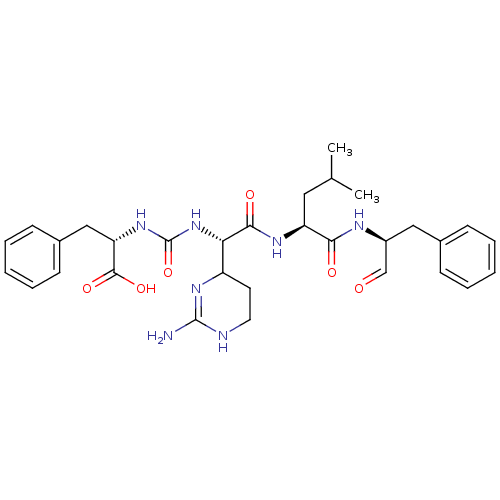 (CHEMBL247767 | Chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description inhibitory activity was evaluated against chymase from human heart. | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM87059 (CHEMBL247767 | Chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098882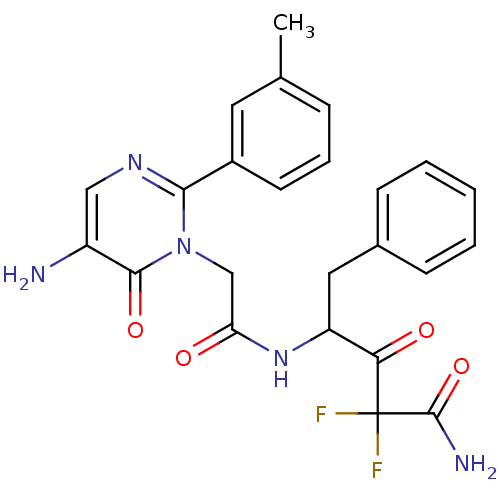 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098877 (4-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068912 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-4-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098849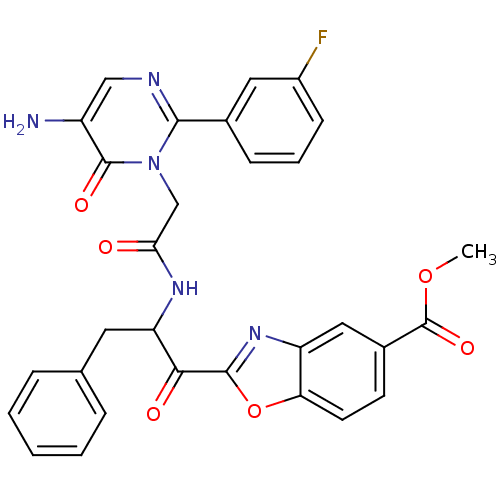 (2-(2-{2-[5-Amino-2-(3-fluoro-phenyl)-6-oxo-6H-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity evaluated against chymase from human heart. | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098876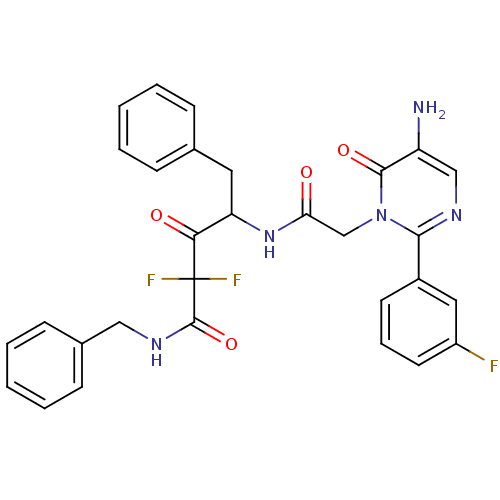 (4-{2-[5-Amino-2-(3-fluoro-phenyl)-6-oxo-6H-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068908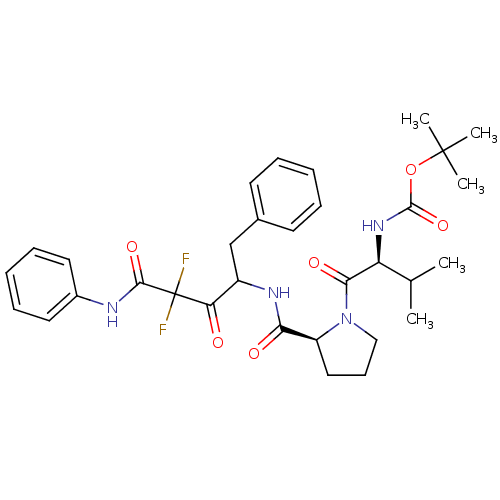 (CHEMBL352917 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 137 total ) | Next | Last >> |