

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Bos taurus (bovine)) | BDBM16499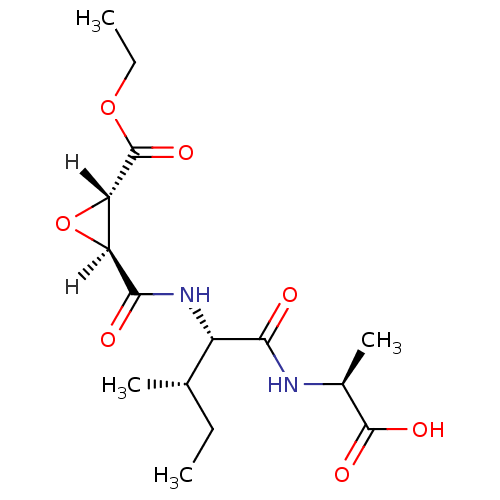 ((2S)-2-[(2S,3S)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16500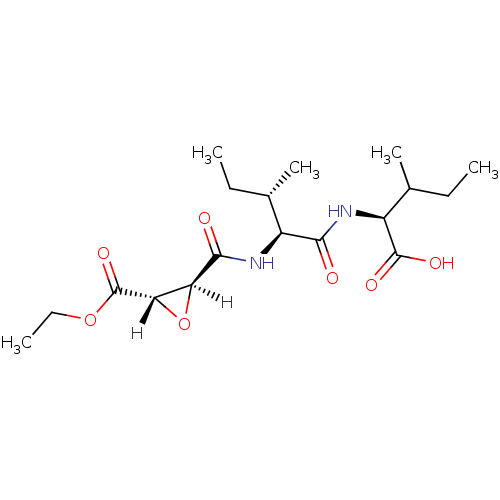 ((2S)-2-[(2S,3S)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16502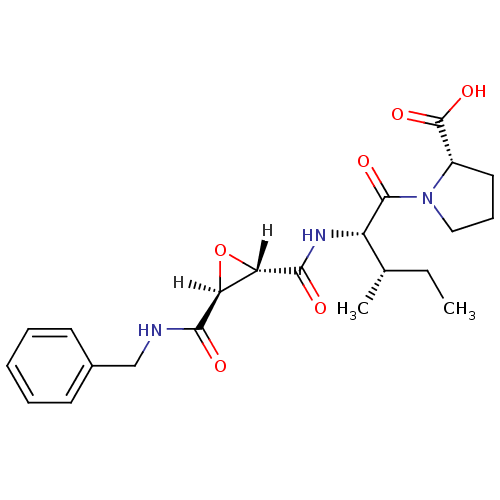 ((2S)-1-[(2S,3S)-2-{[(2S,3S)-3-(benzylcarbamoyl)oxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16509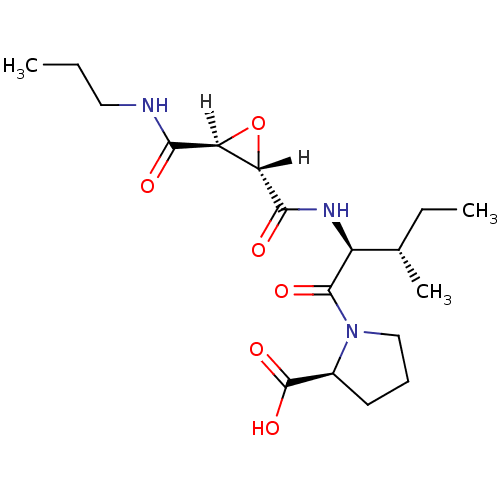 ((2S)-1-[(2S,3S)-3-methyl-2-{[(2S,3S)-3-(propylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16510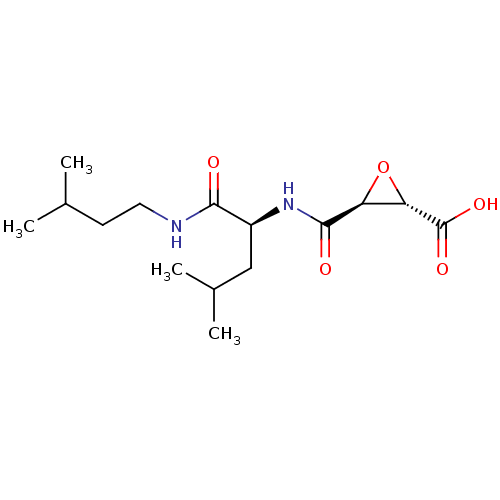 ((2S,3S)-3-[[(1S)-1-(isoamylcarbamoyl)-3-methyl-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046899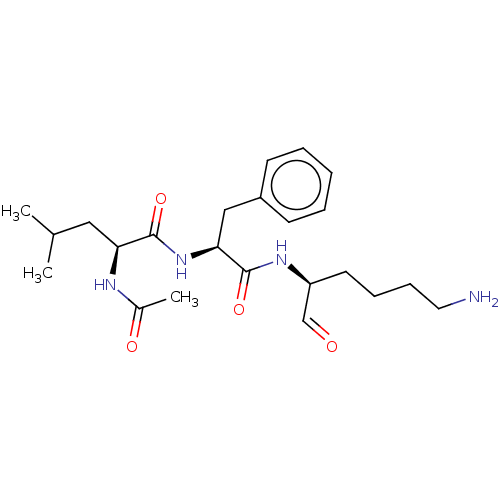 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16508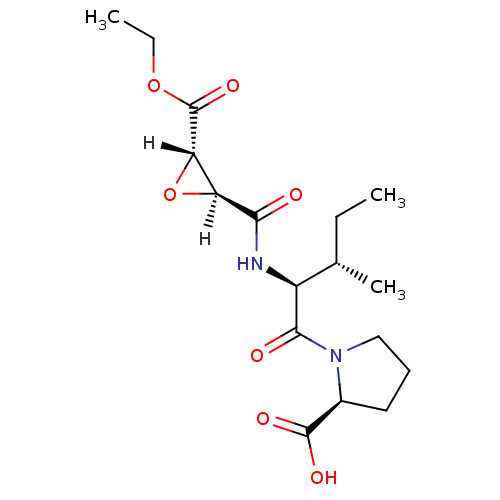 ((2S)-1-[(2S,3S)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16501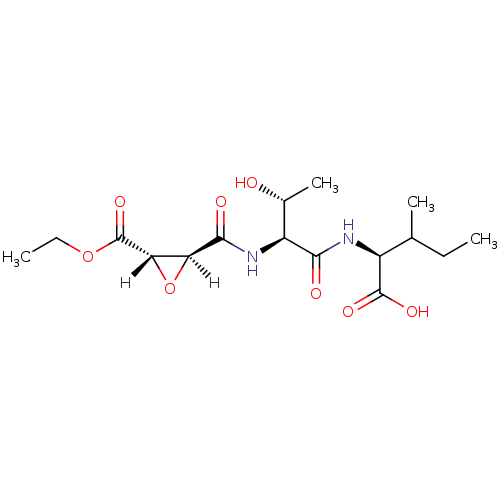 ((2S)-2-[(2S,3R)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046896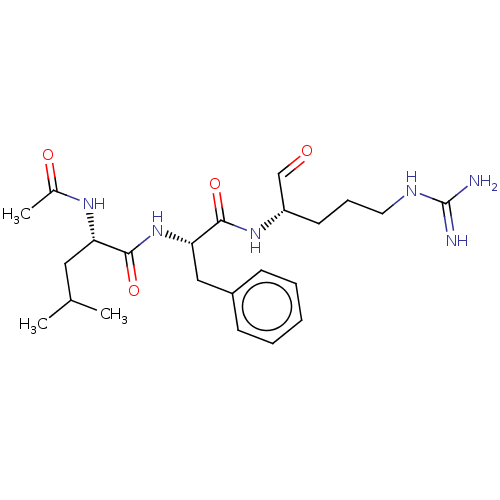 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046887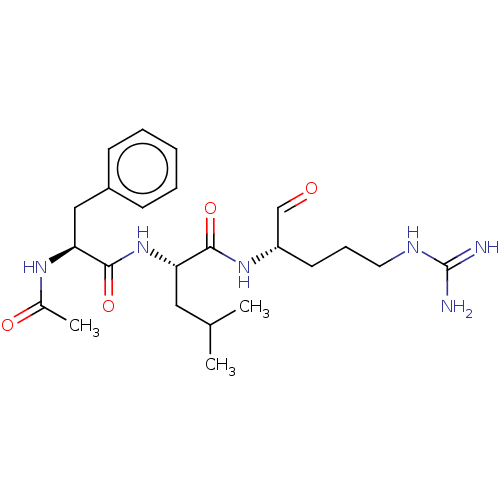 (2-(2-Acetylamino-3-phenyl-propionylamino)-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046888 (2-(2-Acetylamino-3-methyl-butyrylamino)-4-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50484453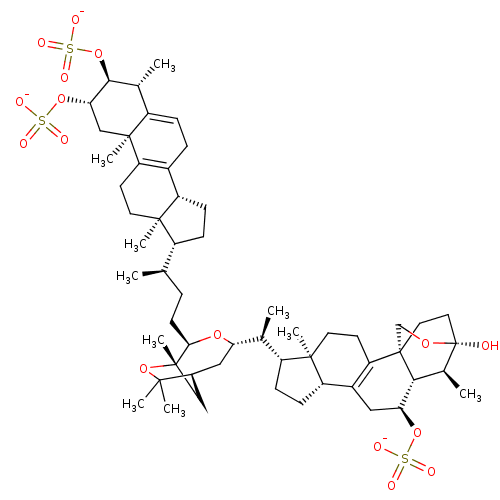 (Shishicrellastatin A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of bovine cathepsin B after 1 hr by fluorescence assay | Bioorg Med Chem 19: 6594-8 (2011) Article DOI: 10.1016/j.bmc.2011.06.052 BindingDB Entry DOI: 10.7270/Q2PN98GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50484452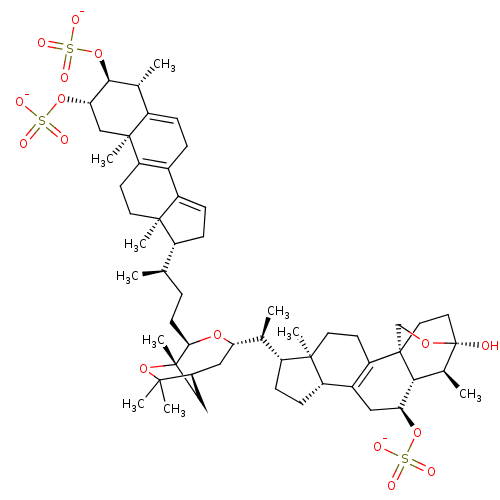 (Shishicrellastatin B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of bovine cathepsin B after 1 hr by fluorescence assay | Bioorg Med Chem 19: 6594-8 (2011) Article DOI: 10.1016/j.bmc.2011.06.052 BindingDB Entry DOI: 10.7270/Q2PN98GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16504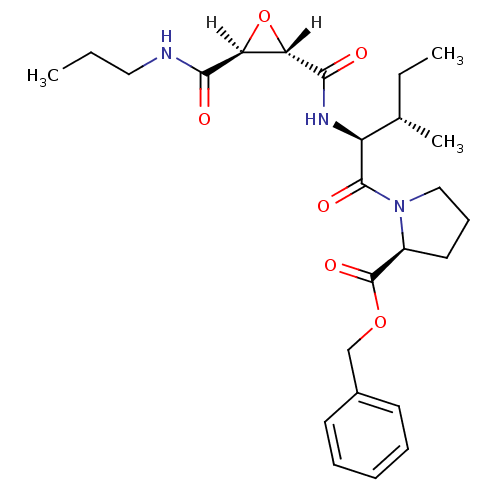 (CA inhibitor 7 | CA073 | PrNH-tES-Ile-Pro-OBzl | b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50047352 (CHEMBL3314610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of bovine spleen cathepsin B using Z-Arg-Arg-AMC as substrate after 30 mins by fluorescence assay | J Nat Prod 77: 1749-52 (2014) Article DOI: 10.1021/np500337m BindingDB Entry DOI: 10.7270/Q2W37XXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046885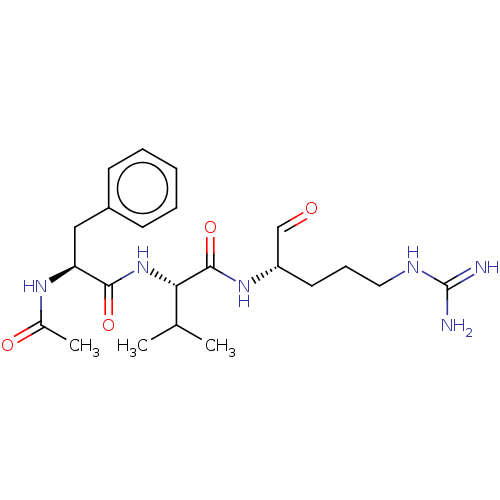 (2-(2-Acetylamino-3-phenyl-propionylamino)-N-(1-for...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046890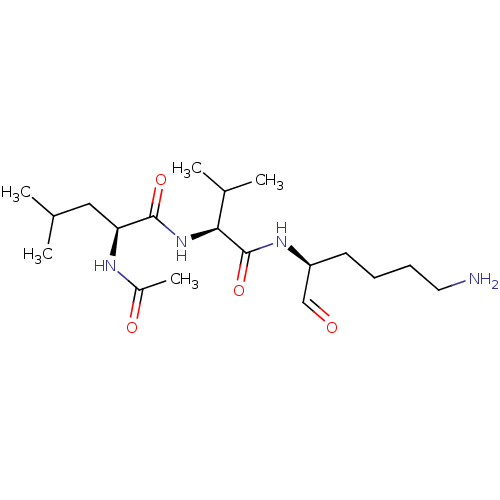 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50047353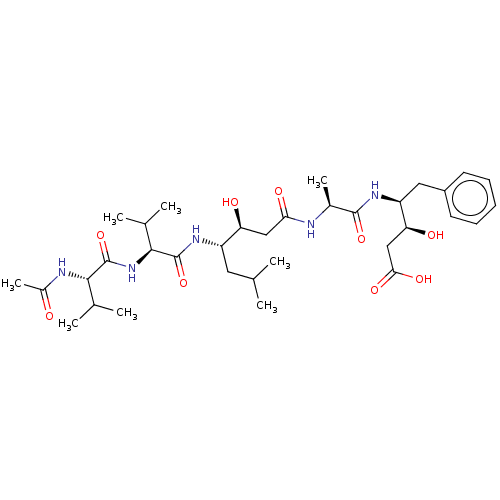 (CHEMBL3314607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of bovine spleen cathepsin B using Z-Arg-Arg-AMC as substrate after 30 mins by fluorescence assay | J Nat Prod 77: 1749-52 (2014) Article DOI: 10.1021/np500337m BindingDB Entry DOI: 10.7270/Q2W37XXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16506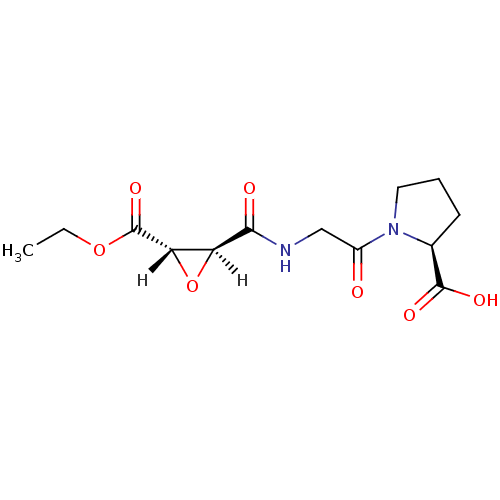 ((2S)-1-(2-{[(2S,3S)-3-(ethoxycarbonyl)oxiran-2-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50368642 (ACETYLPEPSTATIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of bovine spleen cathepsin B using Z-Arg-Arg-AMC as substrate after 30 mins by fluorescence assay | J Nat Prod 77: 1749-52 (2014) Article DOI: 10.1021/np500337m BindingDB Entry DOI: 10.7270/Q2W37XXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046900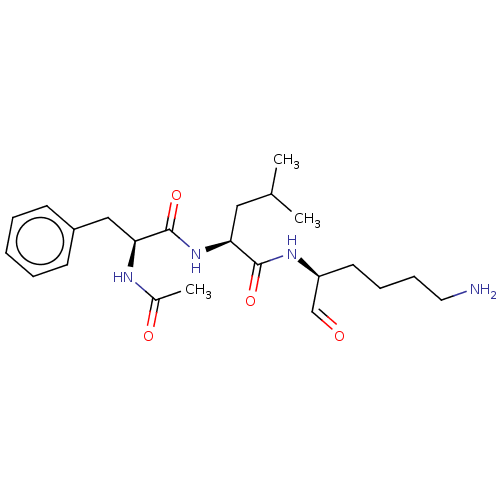 (2-(2-Acetylamino-3-phenyl-propionylamino)-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046891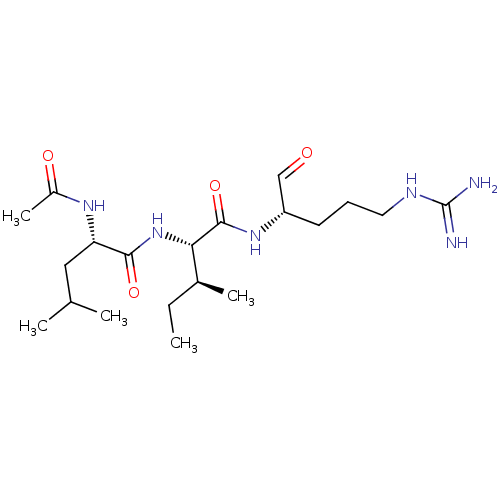 (2-(2-Acetylamino-4-methyl-pentanoylamino)-3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046898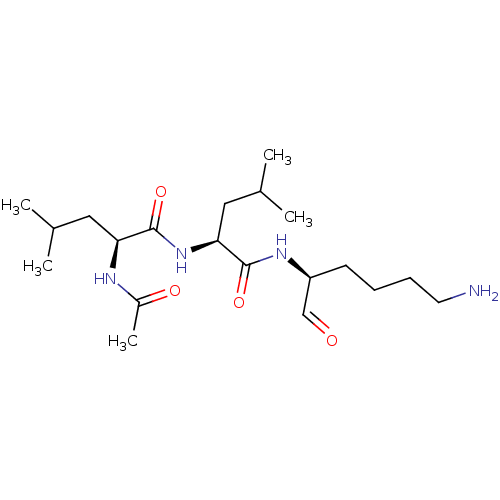 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16497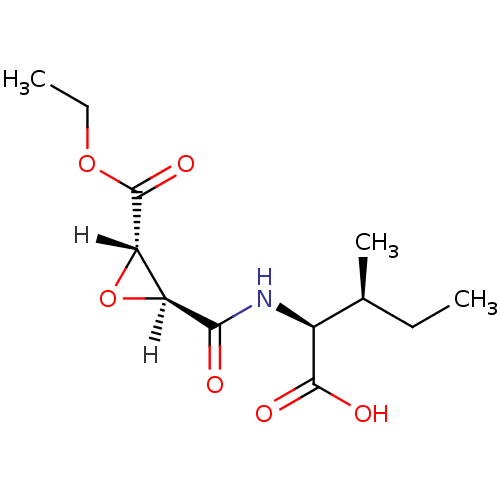 ((2S,3S)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxiran-2-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50047355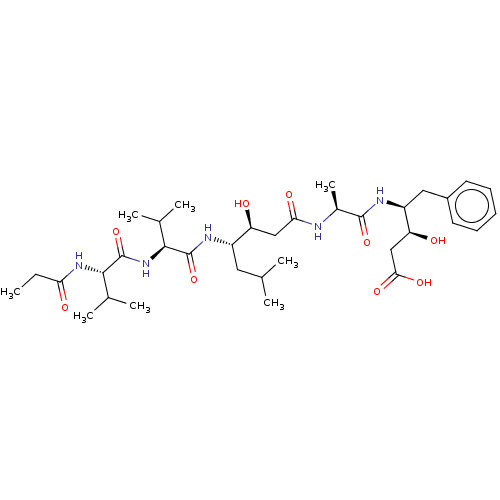 (CHEMBL3314608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of bovine spleen cathepsin B using Z-Arg-Arg-AMC as substrate after 30 mins by fluorescence assay | J Nat Prod 77: 1749-52 (2014) Article DOI: 10.1021/np500337m BindingDB Entry DOI: 10.7270/Q2W37XXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50047354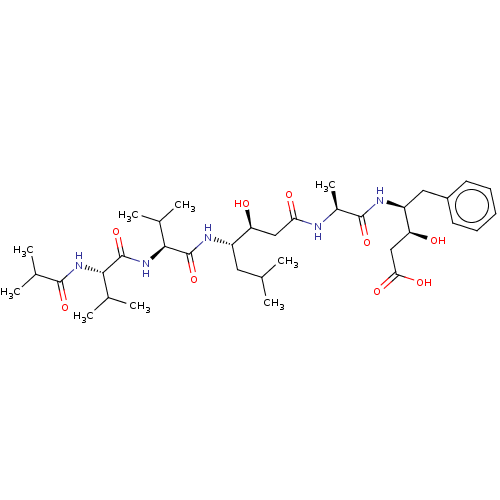 (CHEMBL3314609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of bovine spleen cathepsin B using Z-Arg-Arg-AMC as substrate after 30 mins by fluorescence assay | J Nat Prod 77: 1749-52 (2014) Article DOI: 10.1021/np500337m BindingDB Entry DOI: 10.7270/Q2W37XXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046895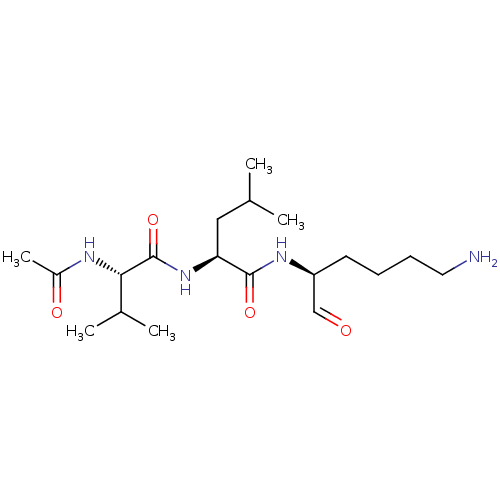 (2-Acetylamino-3-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50047351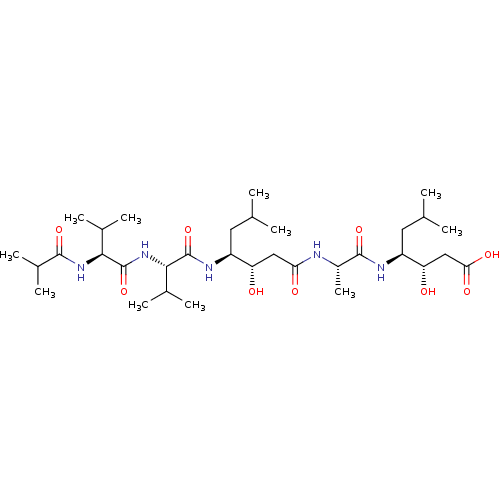 (CHEBI:7988 | CHEMBL3314611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of bovine spleen cathepsin B using Z-Arg-Arg-AMC as substrate after 30 mins by fluorescence assay | J Nat Prod 77: 1749-52 (2014) Article DOI: 10.1021/np500337m BindingDB Entry DOI: 10.7270/Q2W37XXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16505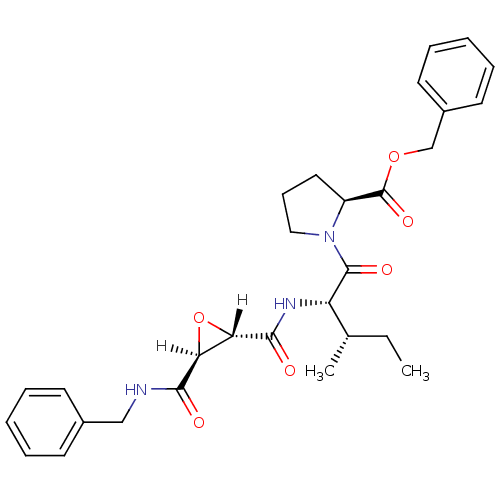 (BzlNH-tES-Ile-Pro-OBzl | CA inhibitor 8 | CA077 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046894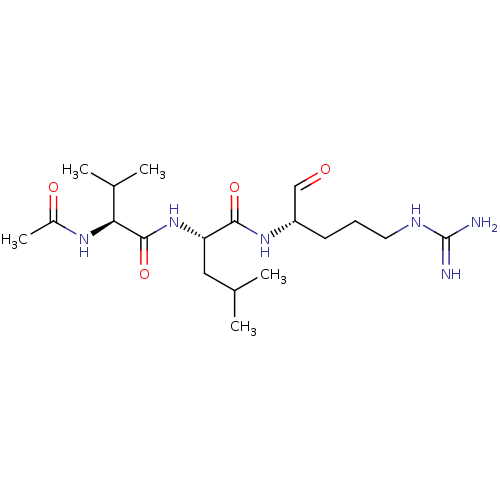 (2-(2-Acetylamino-3-methyl-butyrylamino)-4-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046892 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16503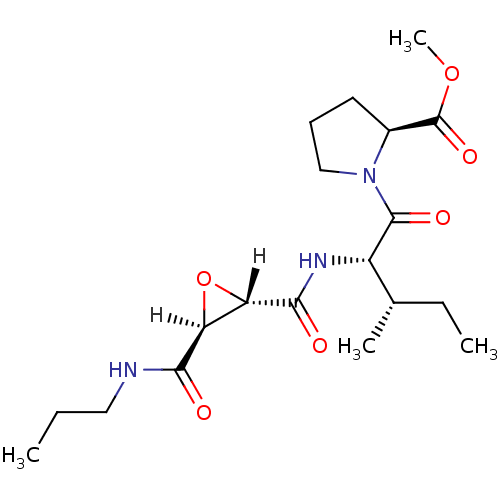 (CA inhibitor 6 | CA074Me | PrNH-tES-Ile-Pro-OMe | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046893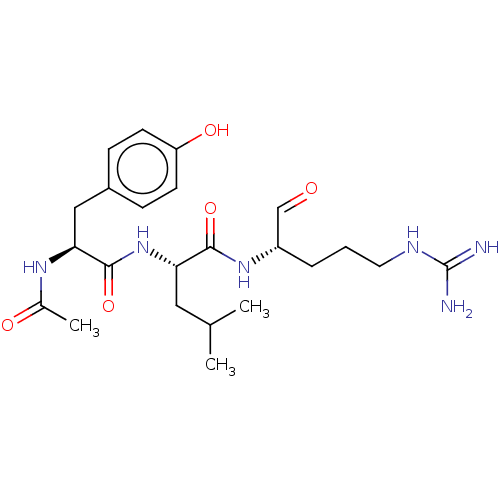 (2-[2-Acetylamino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046889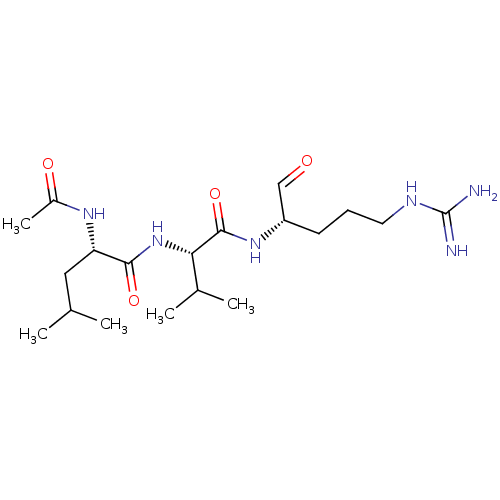 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50147766 (CHEMBL3765121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamagata University Curated by ChEMBL | Assay Description Inhibition of bovine spleen cathepsin B using Z-Arg-Arg-MCA as substrate incubated for 10 mins | Bioorg Med Chem 24: 1241-54 (2016) Article DOI: 10.1016/j.bmc.2016.01.052 BindingDB Entry DOI: 10.7270/Q23T9K25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50224807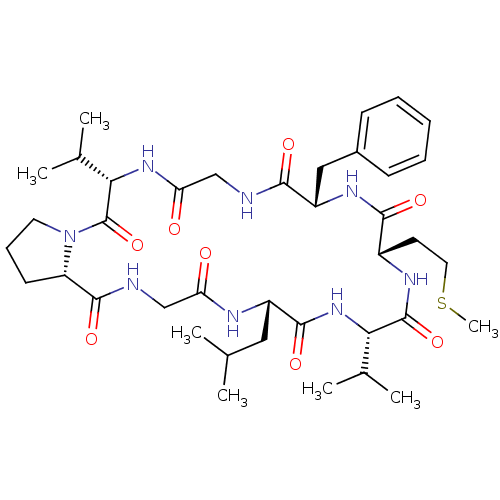 (CHEMBL400366 | cyclo(Pro-Gly-Leu-Val-Met-Phe-Gly-V...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Inhibition of bovine Cathepsin B | J Nat Prod 70: 1611-5 (2007) Article DOI: 10.1021/np0700873 BindingDB Entry DOI: 10.7270/Q23N233F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50147767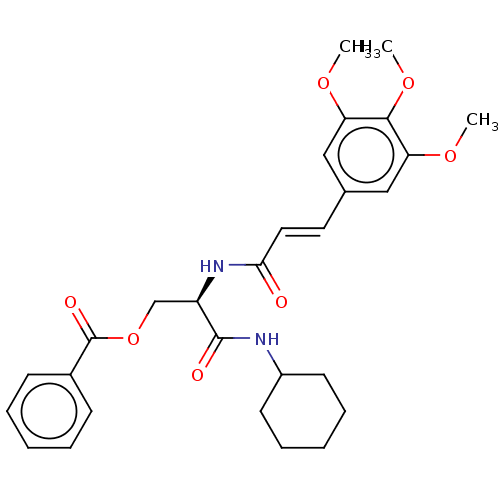 (CHEMBL3765089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamagata University Curated by ChEMBL | Assay Description Inhibition of bovine spleen cathepsin B using Z-Arg-Arg-MCA as substrate incubated for 10 mins | Bioorg Med Chem 24: 1241-54 (2016) Article DOI: 10.1016/j.bmc.2016.01.052 BindingDB Entry DOI: 10.7270/Q23T9K25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||