

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947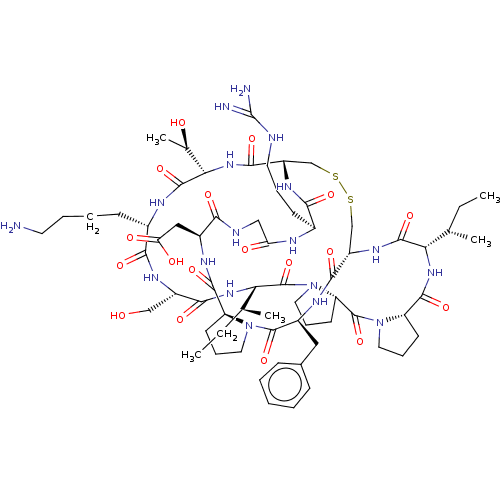 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using Boc-QAR-MCA as substrate measured every 30 secs for 10 mins | Eur J Med Chem 155: 695-704 (2018) Article DOI: 10.1016/j.ejmech.2018.06.029 BindingDB Entry DOI: 10.7270/Q29026CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50452162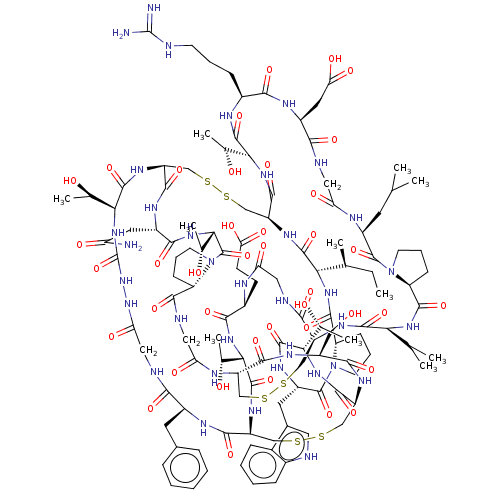 (CHEMBL4212172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin incubated for 5 mins using BAPNA as substrate by colorimetric assay | Bioorg Med Chem Lett 27: 5089-5099 (2017) Article DOI: 10.1016/j.bmcl.2017.10.051 BindingDB Entry DOI: 10.7270/Q2NK3HMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50452161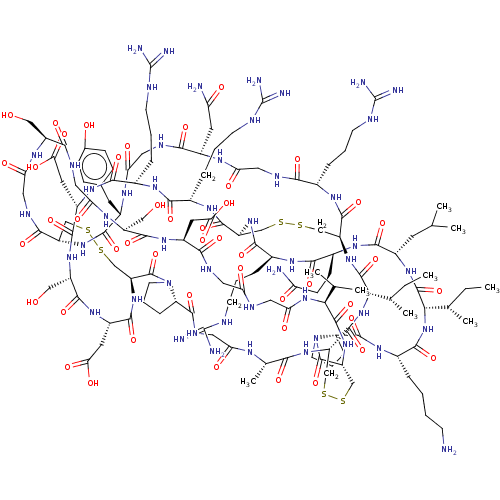 (CHEMBL4217607) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin incubated for 5 mins using BAPNA as substrate by colorimetric assay | Bioorg Med Chem Lett 27: 5089-5099 (2017) Article DOI: 10.1016/j.bmcl.2017.10.051 BindingDB Entry DOI: 10.7270/Q2NK3HMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50452160 (CHEMBL4217569) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin incubated for 5 mins using BAPNA as substrate by colorimetric assay | Bioorg Med Chem Lett 27: 5089-5099 (2017) Article DOI: 10.1016/j.bmcl.2017.10.051 BindingDB Entry DOI: 10.7270/Q2NK3HMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50452159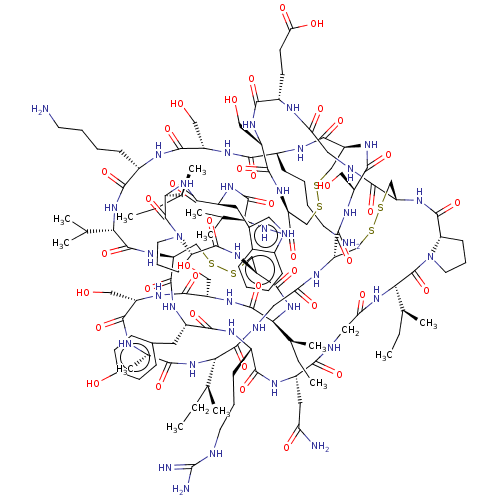 (CHEMBL4210529) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin incubated for 5 mins using BAPNA as substrate by colorimetric assay | Bioorg Med Chem Lett 27: 5089-5099 (2017) Article DOI: 10.1016/j.bmcl.2017.10.051 BindingDB Entry DOI: 10.7270/Q2NK3HMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50452158 (CHEMBL1801042) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin incubated for 5 mins using BAPNA as substrate by colorimetric assay | Bioorg Med Chem Lett 27: 5089-5099 (2017) Article DOI: 10.1016/j.bmcl.2017.10.051 BindingDB Entry DOI: 10.7270/Q2NK3HMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50452156 (CHEMBL4211294) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin incubated for 5 mins using BAPNA as substrate by colorimetric assay | Bioorg Med Chem Lett 27: 5089-5099 (2017) Article DOI: 10.1016/j.bmcl.2017.10.051 BindingDB Entry DOI: 10.7270/Q2NK3HMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076227 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50449678 (CHEMBL4164510) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using Boc-QAR-MCA as substrate measured every 30 secs for 10 mins | Eur J Med Chem 155: 695-704 (2018) Article DOI: 10.1016/j.ejmech.2018.06.029 BindingDB Entry DOI: 10.7270/Q29026CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034581 (CHEMBL36744 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50449677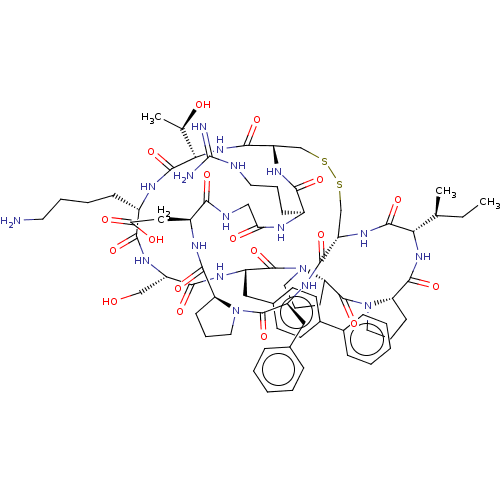 (CHEMBL4172393) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using Boc-QAR-MCA as substrate measured every 30 secs for 10 mins | Eur J Med Chem 155: 695-704 (2018) Article DOI: 10.1016/j.ejmech.2018.06.029 BindingDB Entry DOI: 10.7270/Q29026CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076224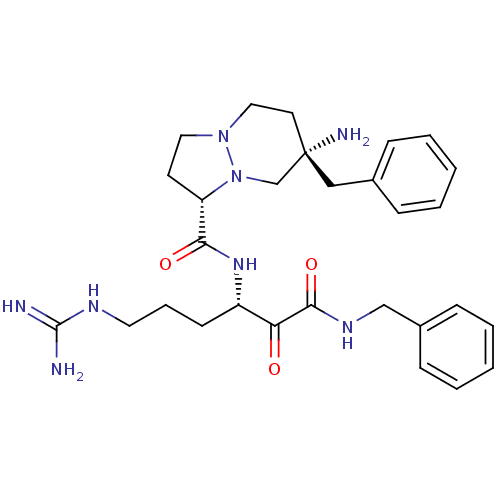 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50502661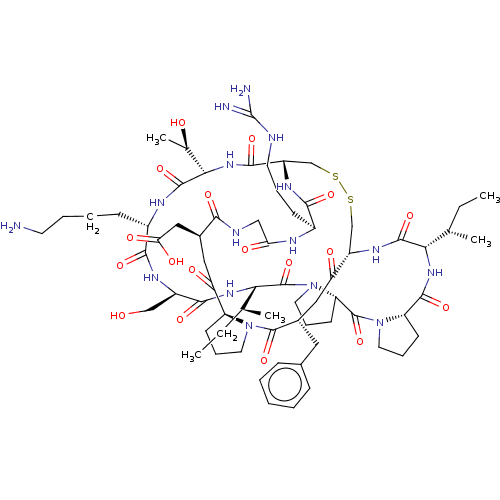 (CHEMBL4531752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using L-BAPNA as substrate preincubated for 5 mins followed by substrate addition and measured for 60 mins by color... | ACS Med Chem Lett 10: 1234-1239 (2019) Article DOI: 10.1021/acsmedchemlett.9b00253 BindingDB Entry DOI: 10.7270/Q2N58QNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50520068 (CHEMBL4447828) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using L-BAPNA as substrate preincubated for 5 mins followed by substrate addition measured after 60 mins | J Med Chem 63: 816-826 (2020) Article DOI: 10.1021/acs.jmedchem.9b01811 BindingDB Entry DOI: 10.7270/Q2RX9GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin type-3 using L-BAPNA as substrate preincubated for 5 mins followed by substrate addition measured over 60 mins | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034585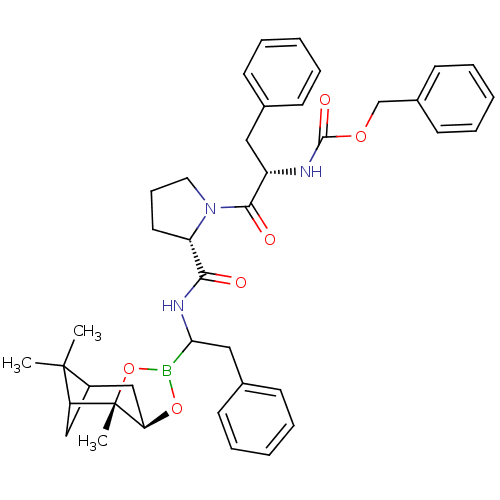 (CHEMBL285285 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50449679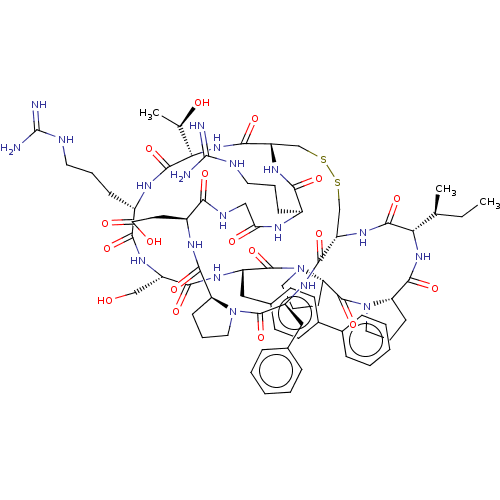 (CHEMBL4167071) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using Boc-QAR-MCA as substrate measured every 30 secs for 10 mins | Eur J Med Chem 155: 695-704 (2018) Article DOI: 10.1016/j.ejmech.2018.06.029 BindingDB Entry DOI: 10.7270/Q29026CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076219 ((3S,6R)-6-Amino-6-benzyl-octahydro-indolizine-3-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50449680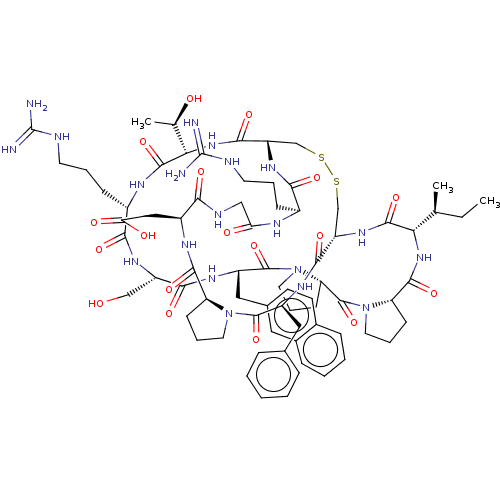 (CHEMBL4170662) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using Boc-QAR-MCA as substrate measured every 30 secs for 10 mins | Eur J Med Chem 155: 695-704 (2018) Article DOI: 10.1016/j.ejmech.2018.06.029 BindingDB Entry DOI: 10.7270/Q29026CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111121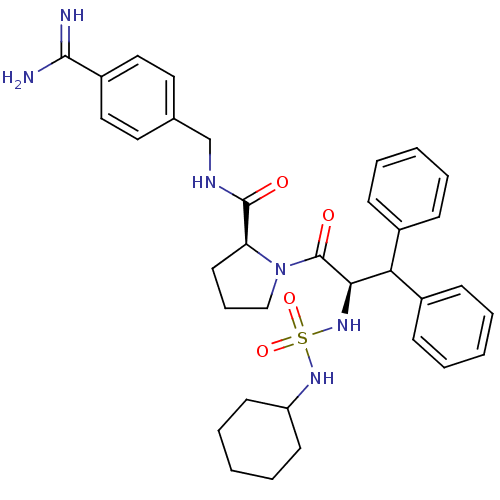 (2N-(4-Benzamidinemethyl)-1-[2-Cyclohexylaminosulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034579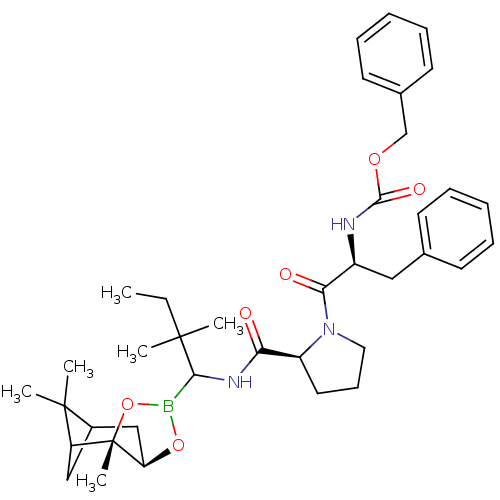 (CHEMBL290577 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14127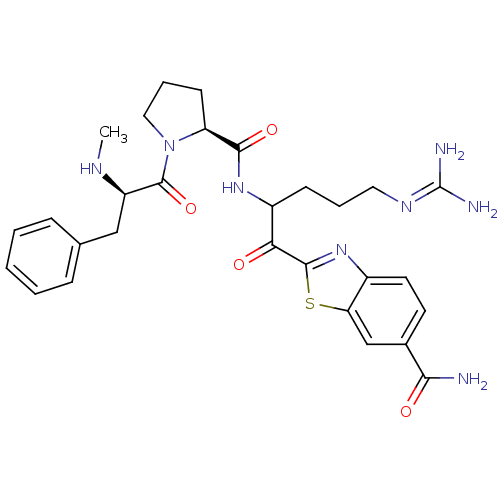 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111110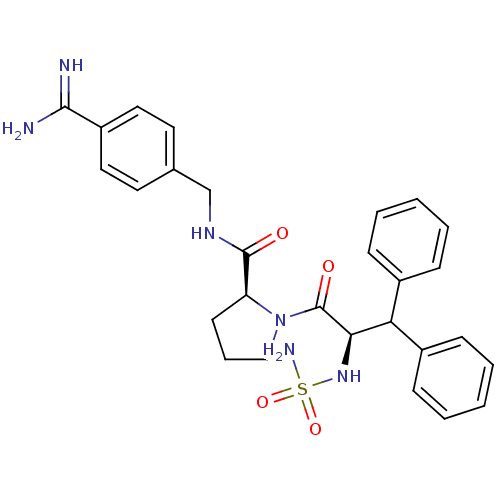 (2N-(4-Benzamidinemethyl)-1-[2-aminosulfonamido-3,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14065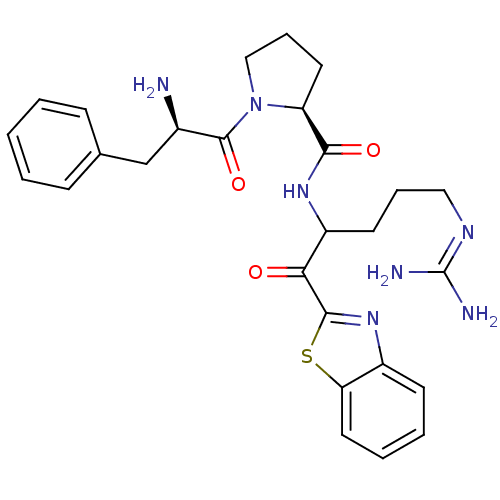 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111122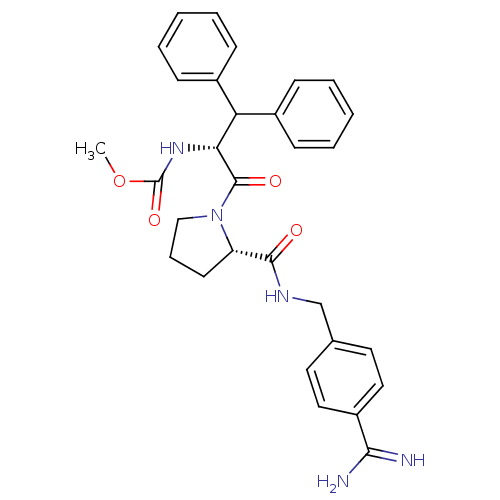 (2N-(4-Benzamidinemethyl)-1-[2-Carbamicacidmethyles...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034577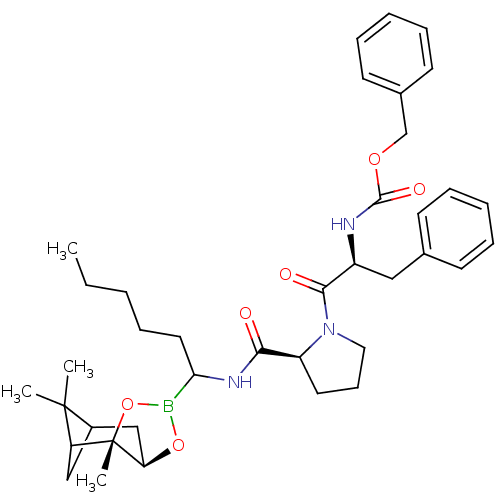 (CHEMBL291026 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034580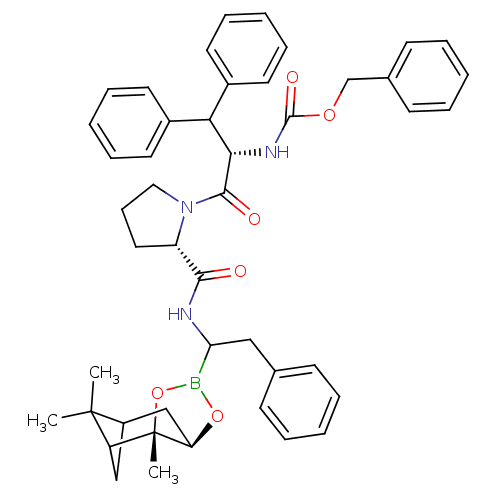 (CHEMBL418050 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034583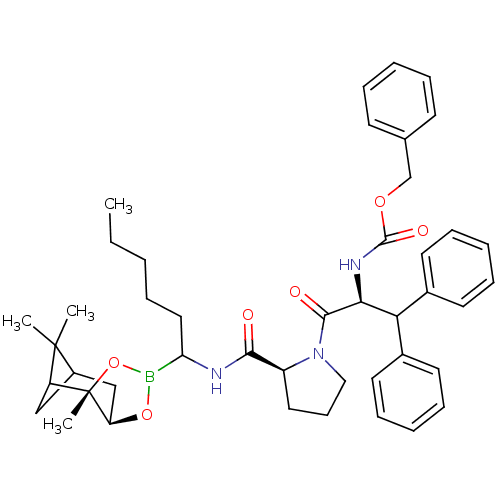 (CHEMBL287918 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034573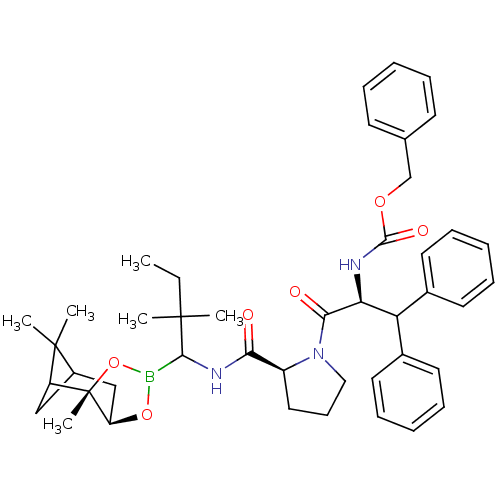 (CHEMBL291261 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111108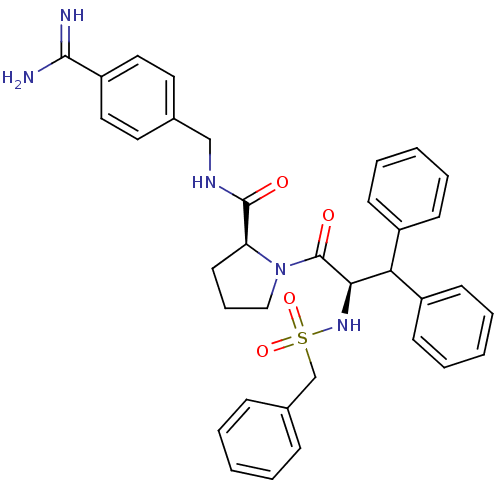 (2N-(4-Benzamidinemethyl)-1-[2-Benzylsulfonamido-3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111105 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076222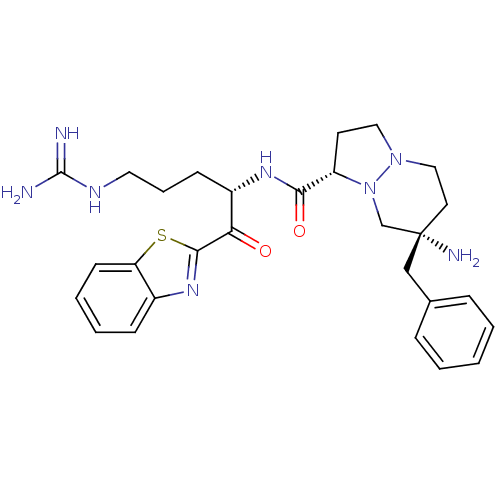 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034574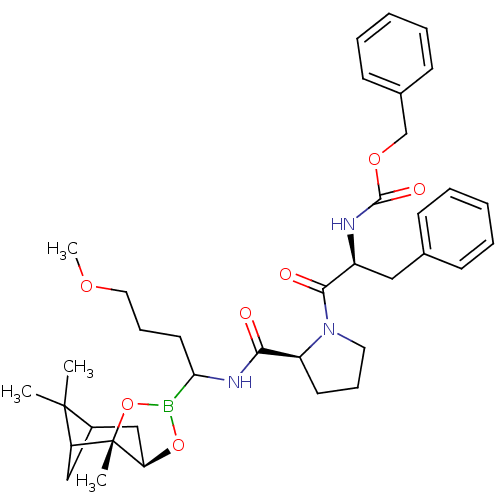 (CHEMBL288150 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111101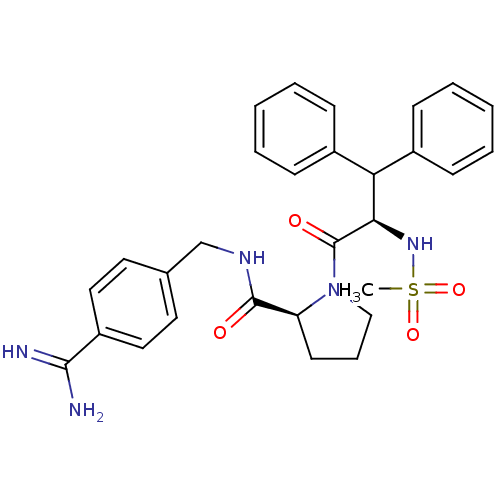 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111103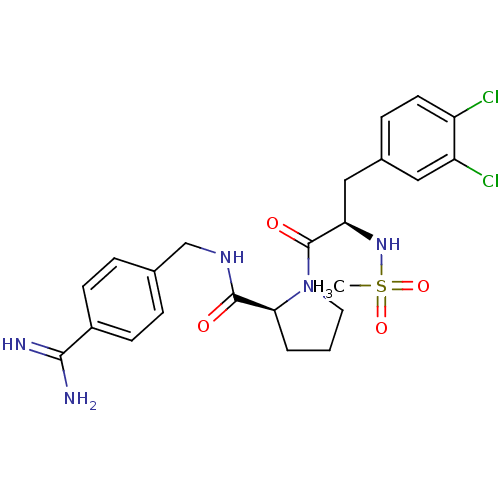 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-methanesulfon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034582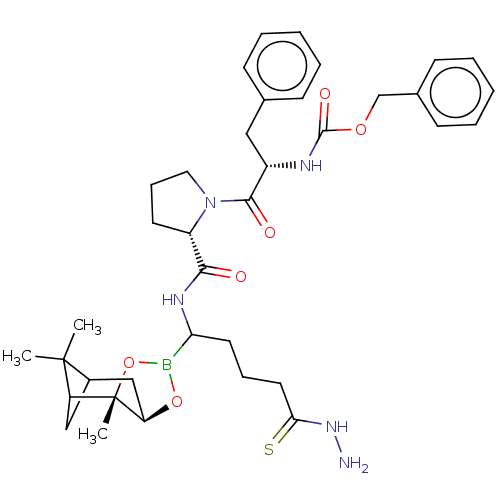 (CHEMBL2448441 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.848 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50288457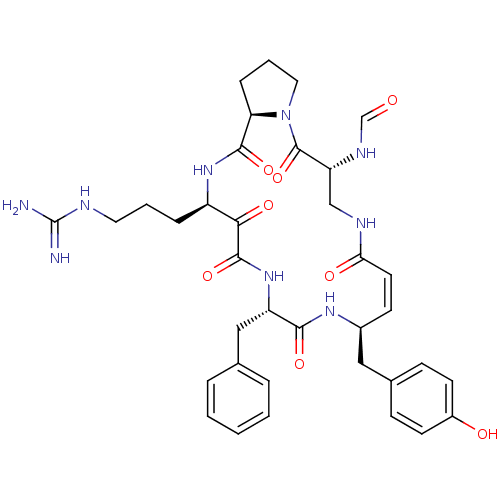 (CHEMBL317974 | macrocyclic tripeptide motif) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of bovine trypsin | Bioorg Med Chem Lett 6: 2947-2952 (1996) Article DOI: 10.1016/S0960-894X(96)00554-9 BindingDB Entry DOI: 10.7270/Q2FX79F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using N-t-Boc Gln-Ala-Arg-AMC as substrate preincubated with enzyme followed by substrate addition by Dixon plot an... | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14117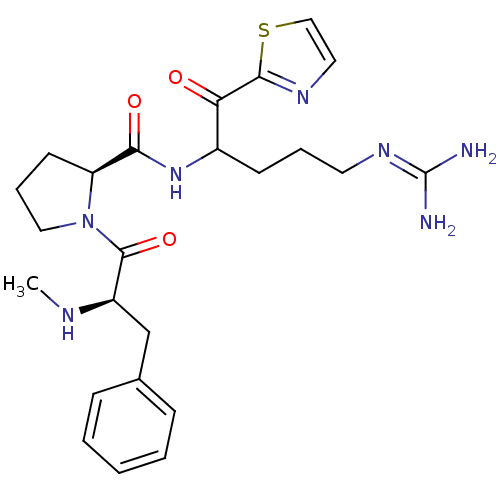 ((2S)-N-[5-carbamimidamido-1-oxo-1-(1,3-thiazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14076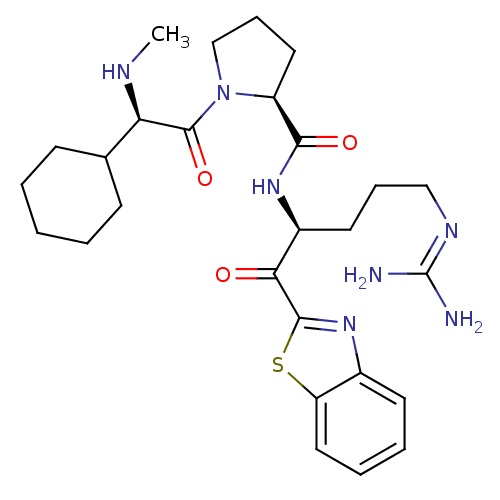 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111102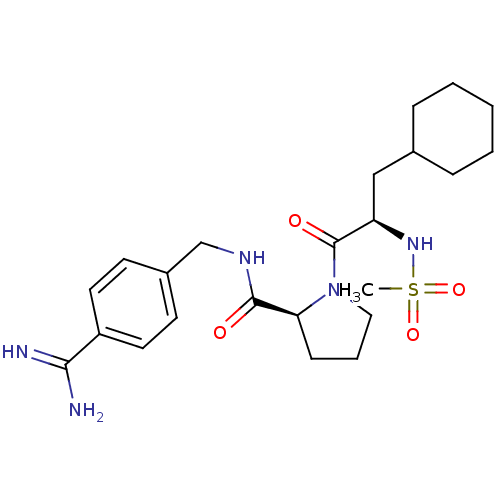 ((S)-1-((R)-3-Cyclohexyl-2-methanesulfonylamino-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14338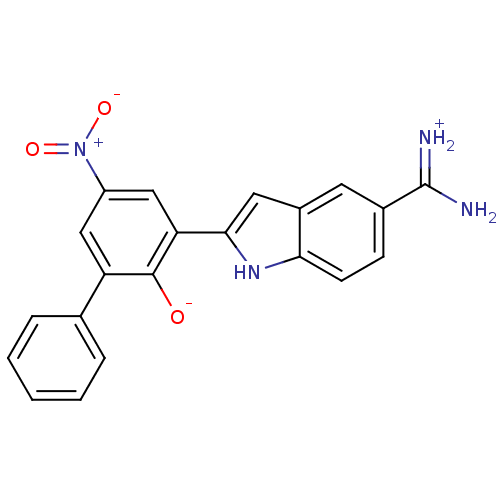 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14085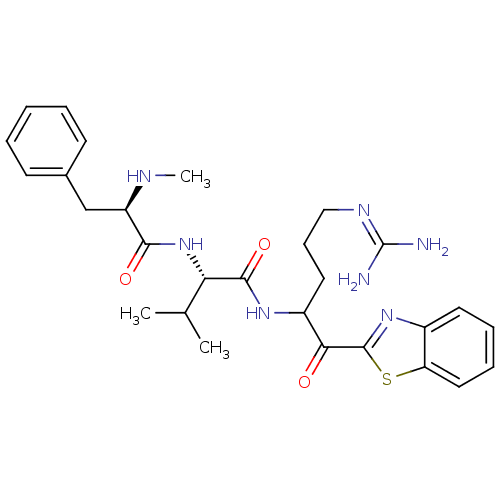 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50111098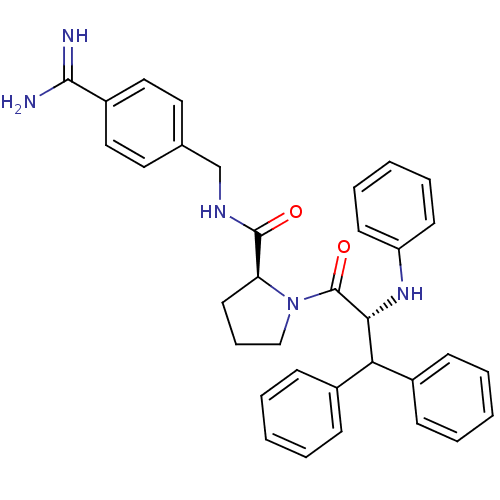 (2N-(4-Benzamidinemethyl)-1-[2-Phenylamino-3,3-diph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14125 ((2S)-N-[5-carbamimidamido-1-(6-fluoro-1,3-benzothi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50052418 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine trypsin | J Med Chem 39: 3039-43 (1996) Article DOI: 10.1021/jm9603274 BindingDB Entry DOI: 10.7270/Q24B3208 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14092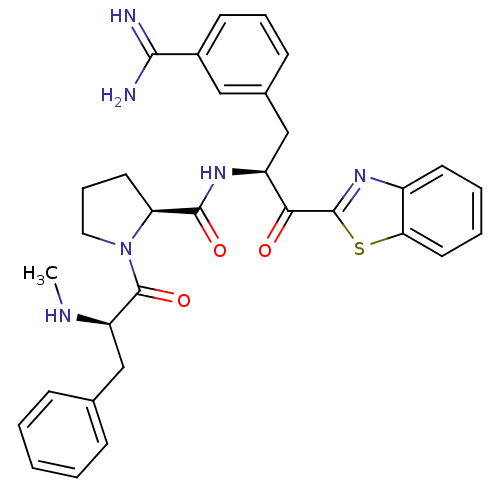 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-3-(3-carbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14086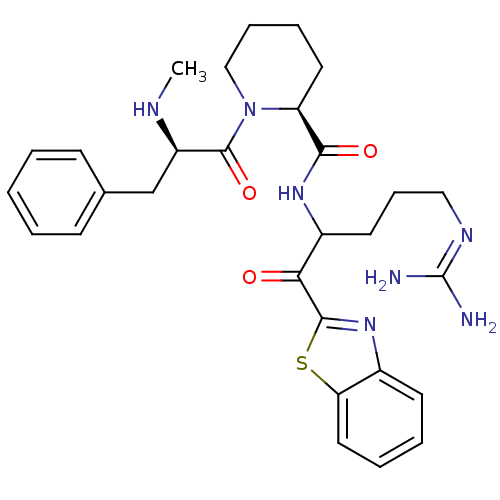 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14078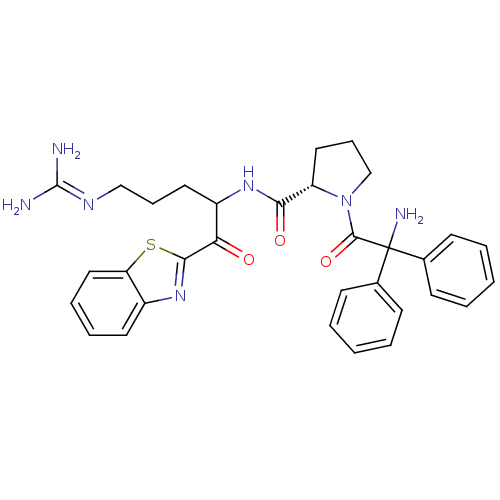 ((2S)-1-(2-amino-2,2-diphenylacetyl)-N-[1-(1,3-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14079 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 999 total ) | Next | Last >> |