

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427622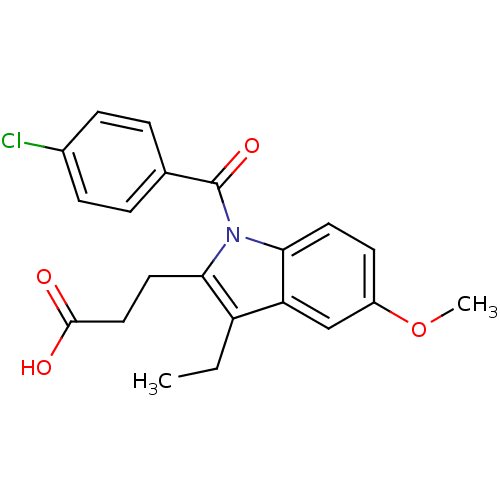 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427628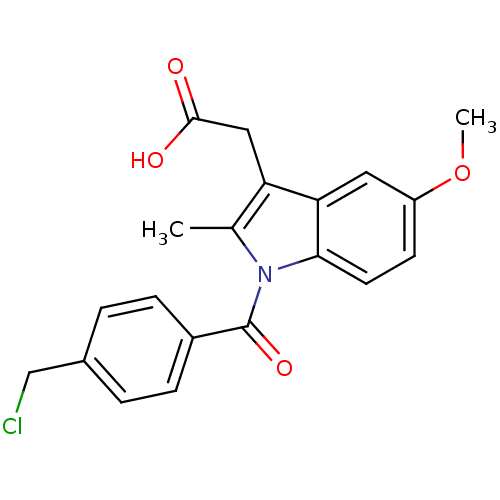 (CHEMBL2323472 | US9346803, Table 2, Compound 8: 2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427620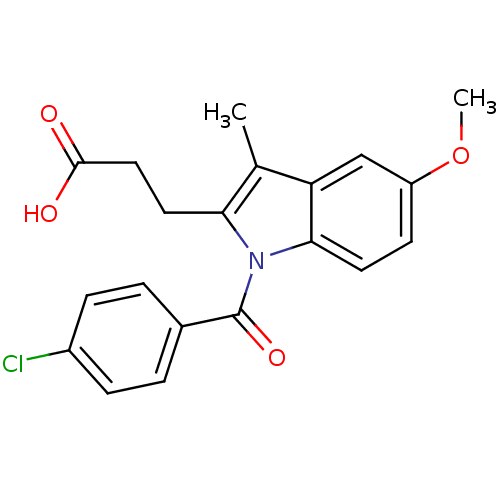 (CHEMBL2323507 | US9346803, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427624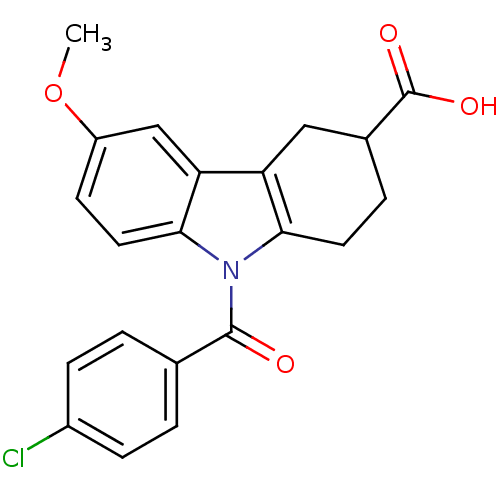 (CHEMBL2323522 | US9346803, Table 2, Compound 11: 9...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427629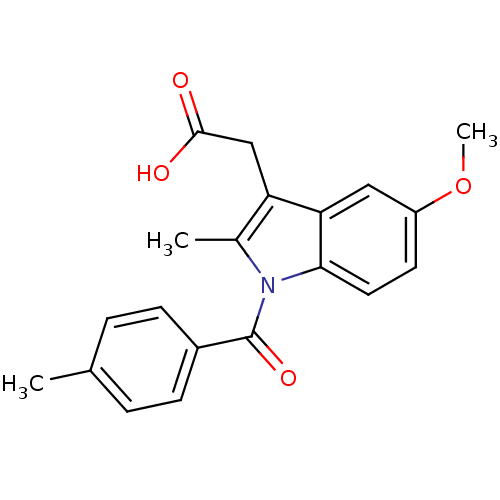 (CHEMBL179587 | US9346803, Table 2, Compound 7: 2-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427621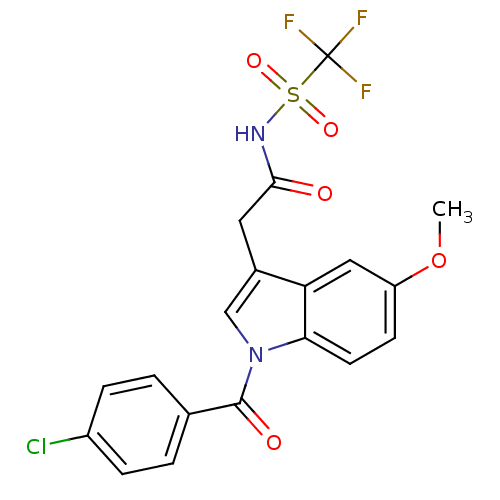 (CHEMBL2323490 | US9346803, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427625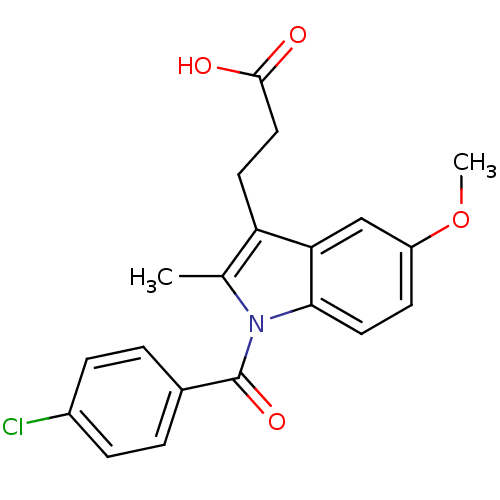 (CHEMBL178687 | US9346803, Table 2, Compound 6: 3-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427627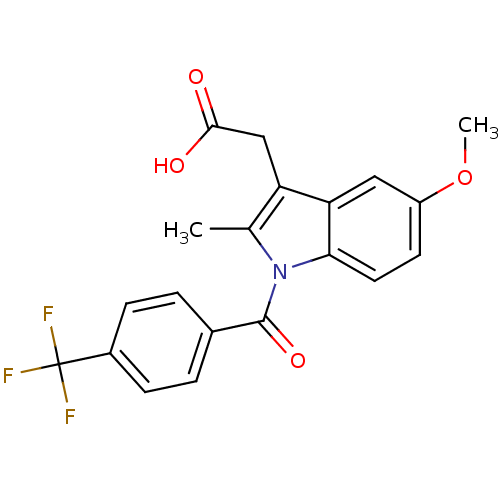 (CHEMBL2323474 | US9346803, Table 2, Compound 9: 2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427619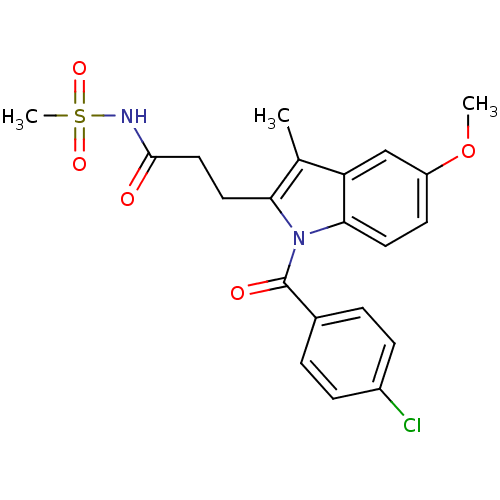 (CHEMBL2323511 | US9346803, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50438582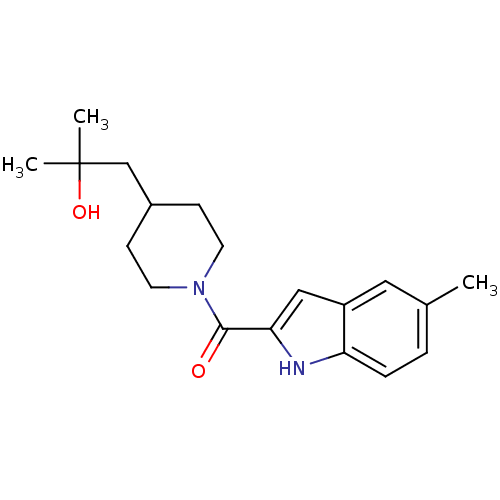 (CHEMBL2413849) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human 17beta-HSD5 expressed in HEK293 cells using androstenedione as substrate assessed as testosterone synthesis after 4 hrs | Bioorg Med Chem 21: 5261-70 (2013) Article DOI: 10.1016/j.bmc.2013.06.025 BindingDB Entry DOI: 10.7270/Q2GF0VXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313838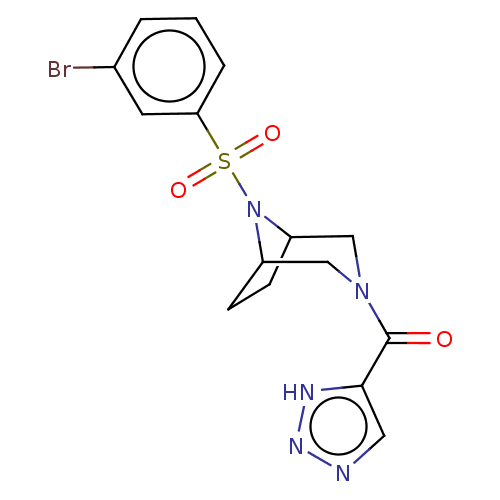 (US10167293, Example 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254841 (US9487554, 28) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313874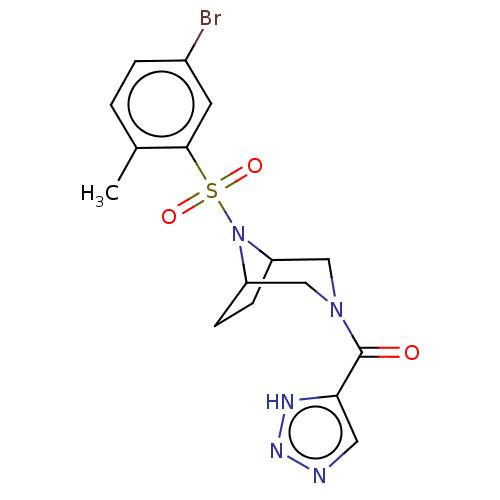 (US10167293, Example 65) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313879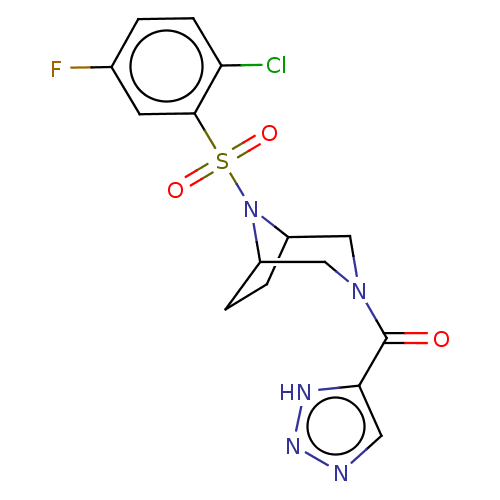 (US10167293, Example 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313837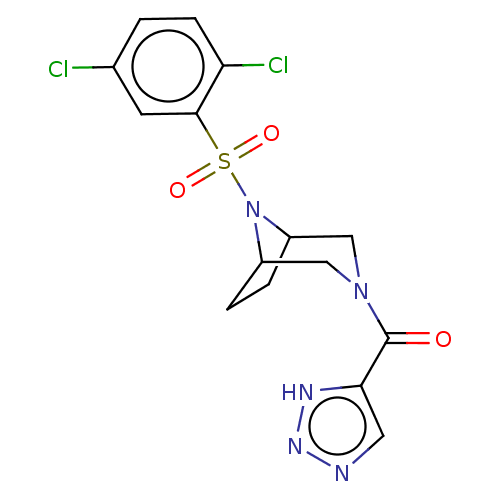 (US10167293, Example 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313878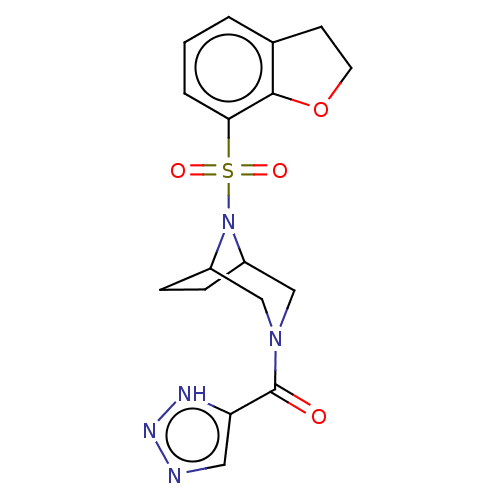 (US10167293, Example 69) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313884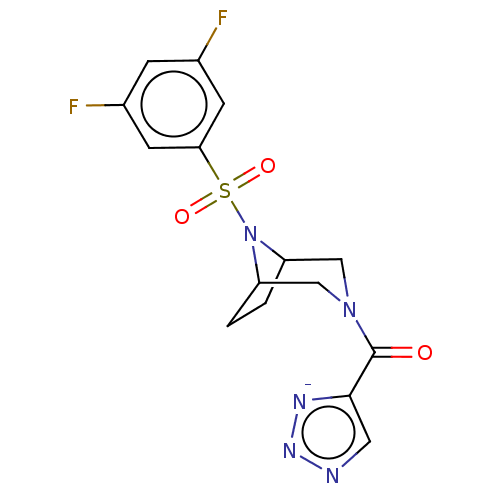 (US10167293, Example 75 | sodium 5-({8-[(3,5-difluo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313839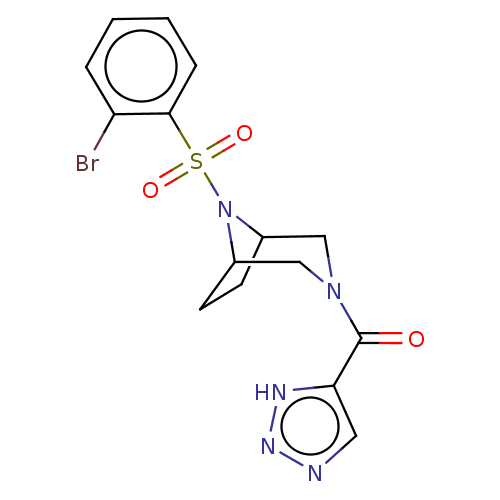 (US10167293, Example 32) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427626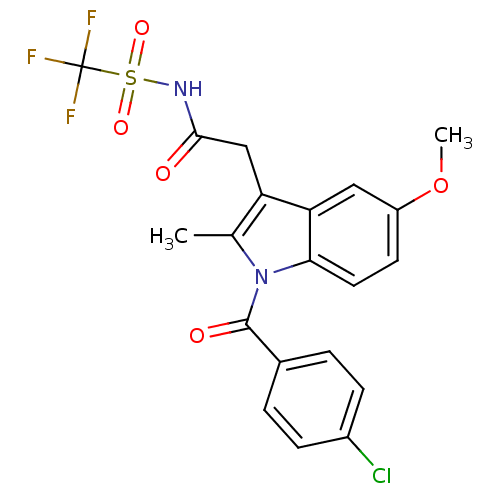 (CHEMBL2323481 | US9346803, Table 2, Compound 5: 2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254826 (US9487554, 13) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313880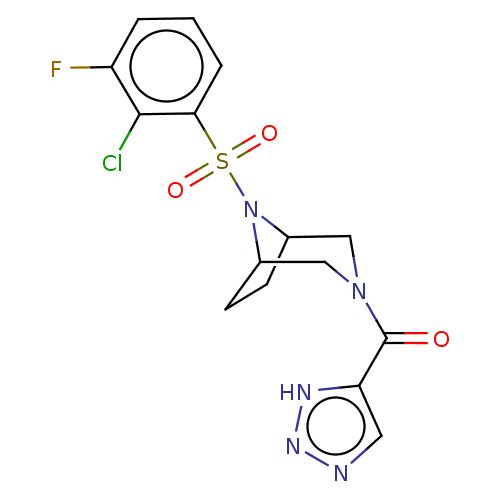 (US10167293, Example 71) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313861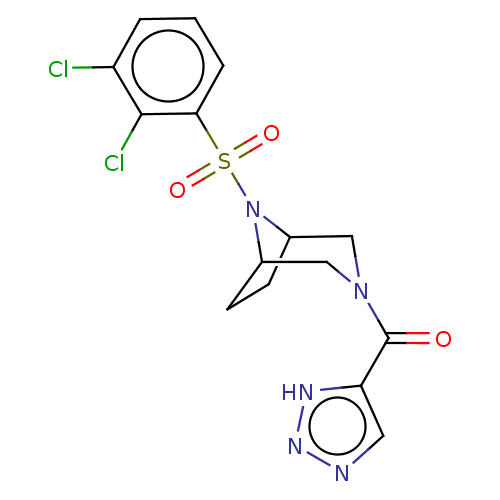 (US10167293, Example 53) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313859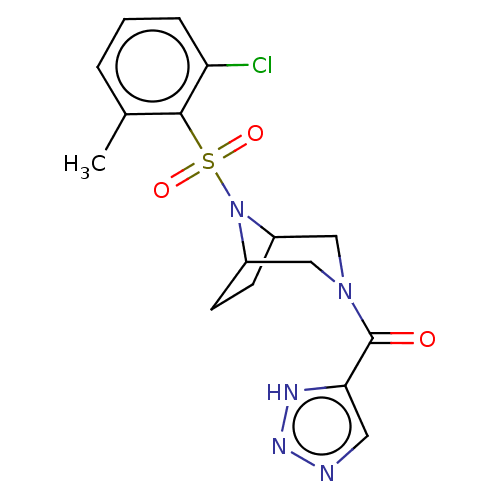 (US10167293, Example 51) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313836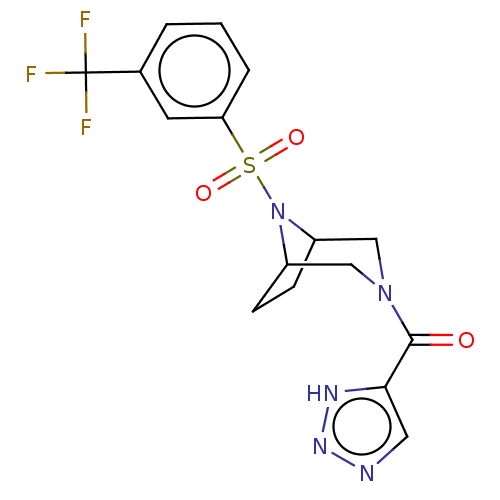 (US10167293, Example 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313875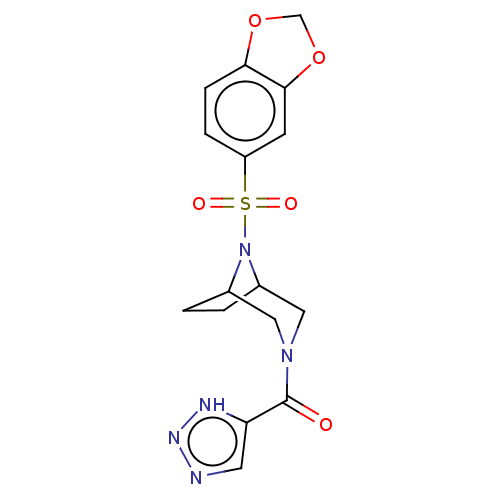 (US10167293, Example 66) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313834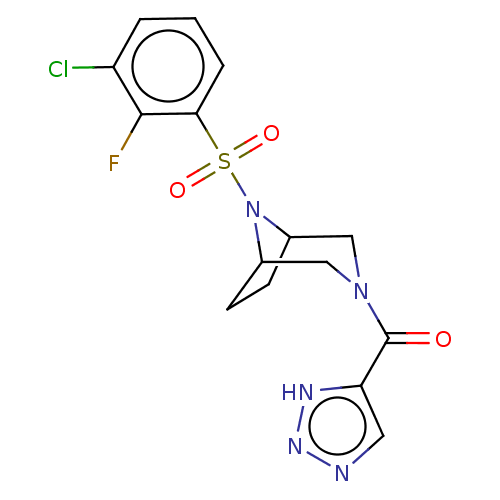 (US10167293, Example 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313816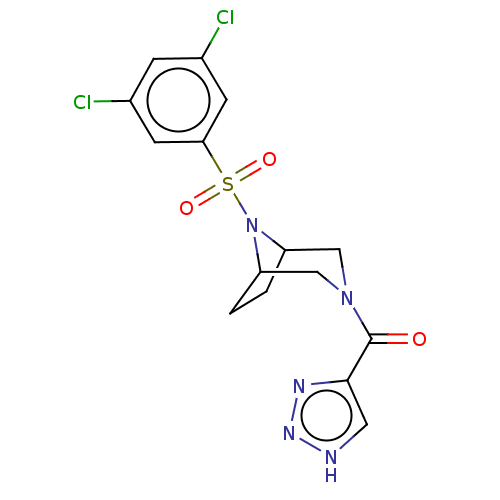 (US10167293, Example 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313858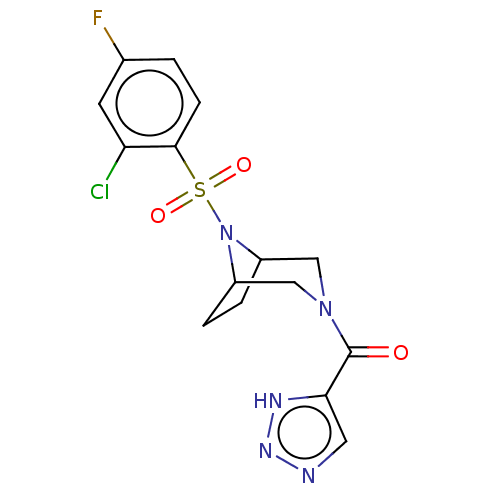 (US10167293, Example 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313854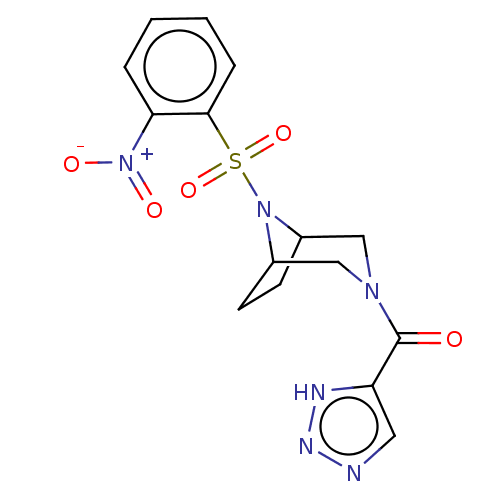 (US10167293, Example 46) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50293598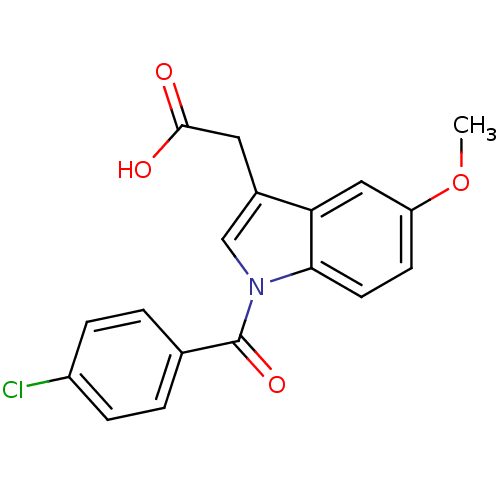 (2'-des-methyl indomethacin | CHEMBL503179 | US9346...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313876 (US10167293, Example 67) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313827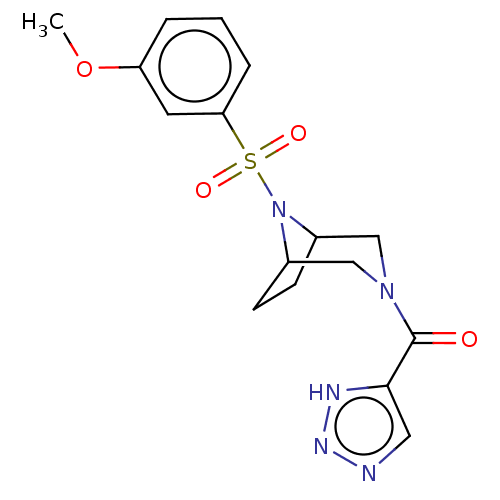 (US10167293, Example 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313809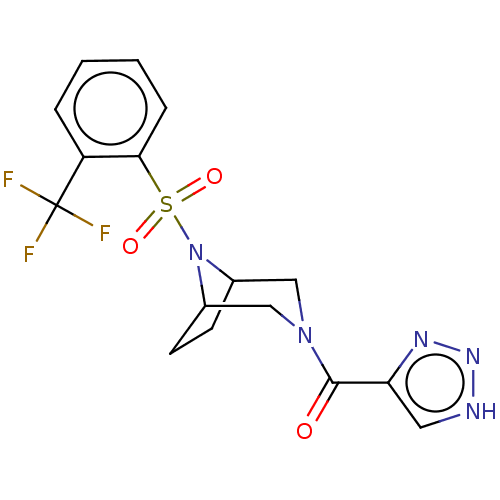 (1H-1,2,3-triazol-4-yl[8-{[2-(trifluoromethyl)pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313825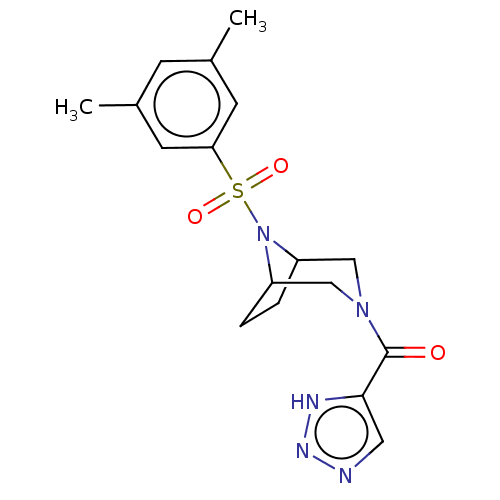 (US10167293, Example 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313881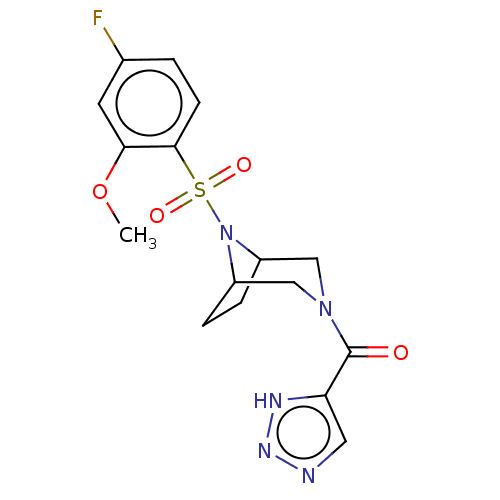 (US10167293, Example 72) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313885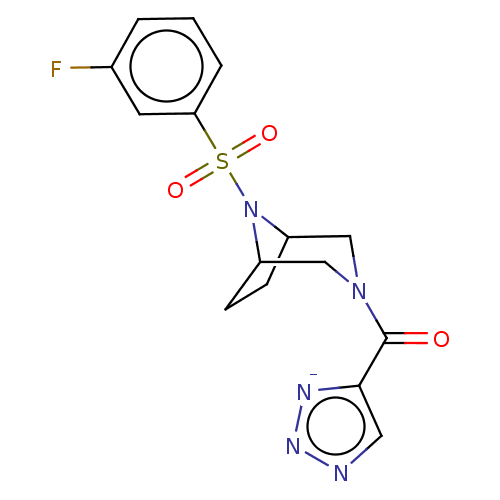 (US10167293, Example 76 | sodium 5-({8-[(3-fluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313856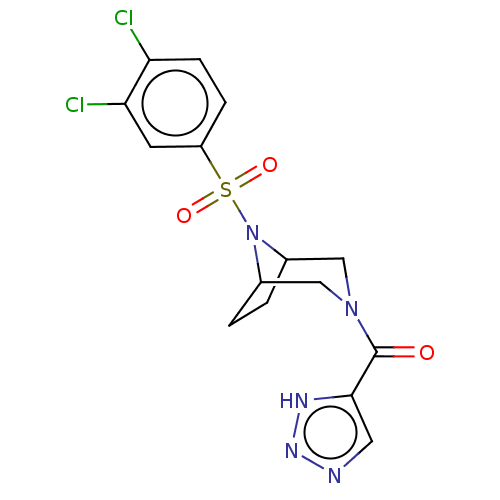 (US10167293, Example 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313850 (US10167293, Example 42) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313826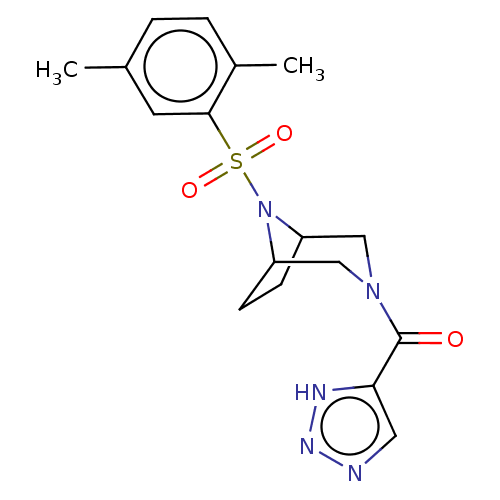 (US10167293, Example 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313819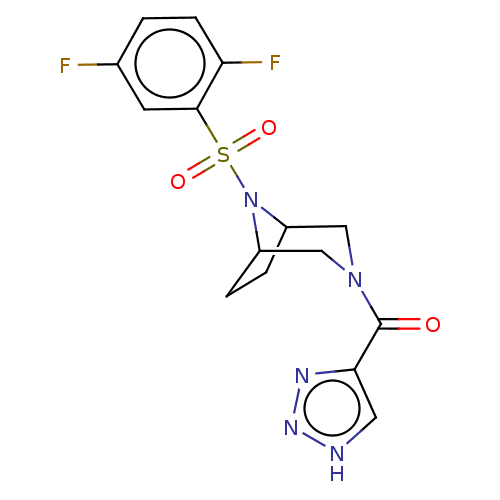 (US10167293, Example 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313818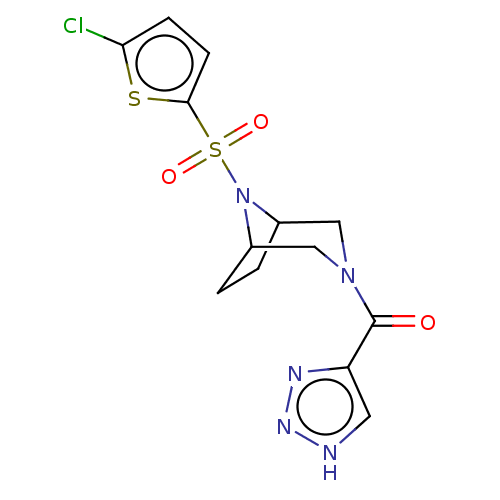 (US10167293, Example 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313833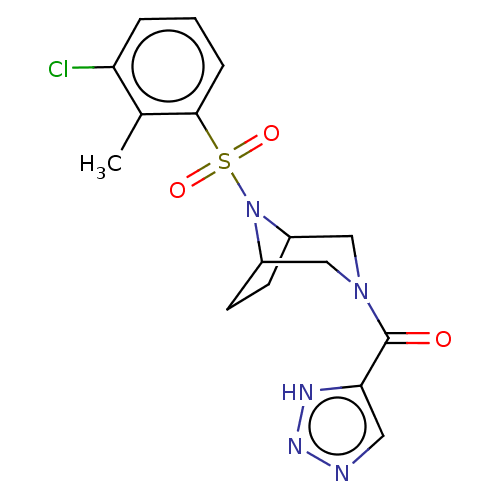 (US10167293, Example 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313812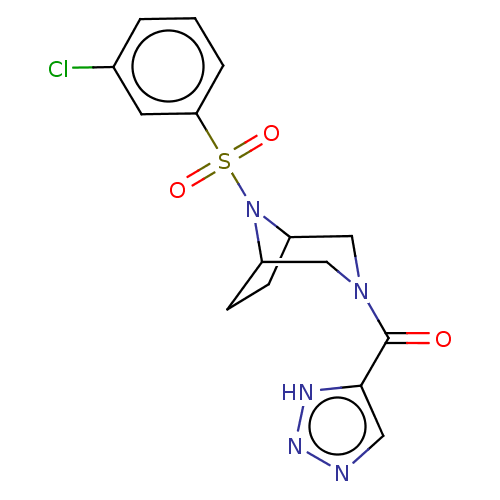 (US10167293, Example 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313811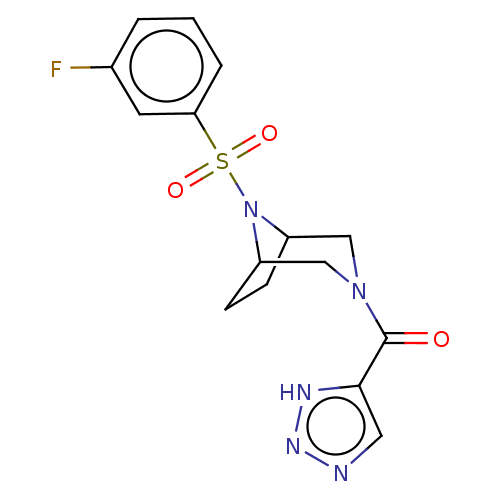 (US10167293, Example 4 | {8-[(3-fluorophenyl)sulfon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313866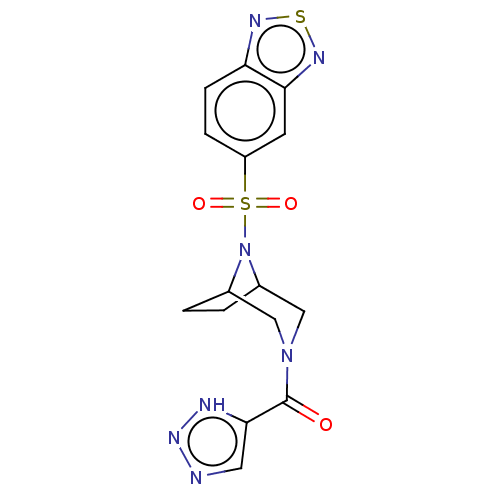 (US10167293, Example 58) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313857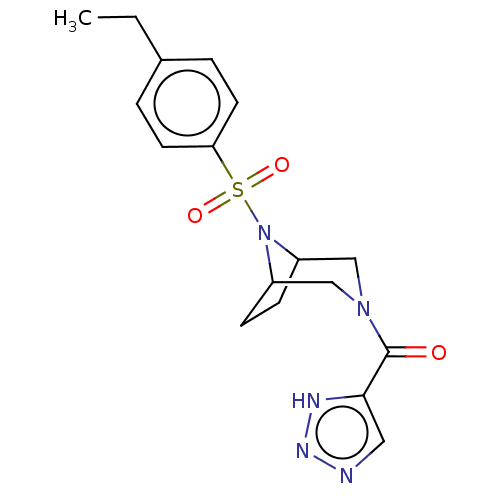 (US10167293, Example 49) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313862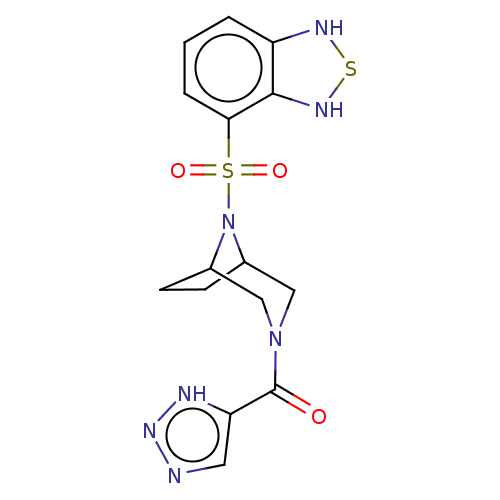 (US10167293, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313835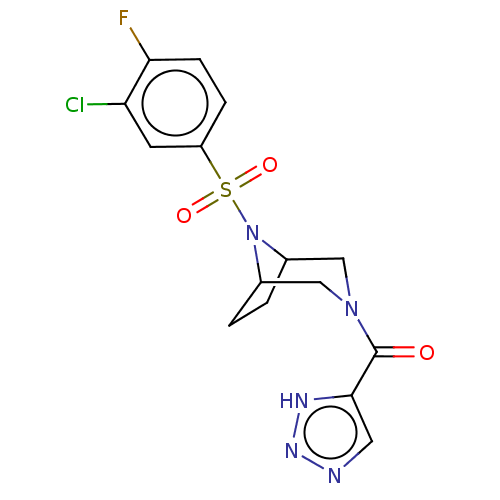 (US10167293, Example 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254825 (US9487554, 12) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM313847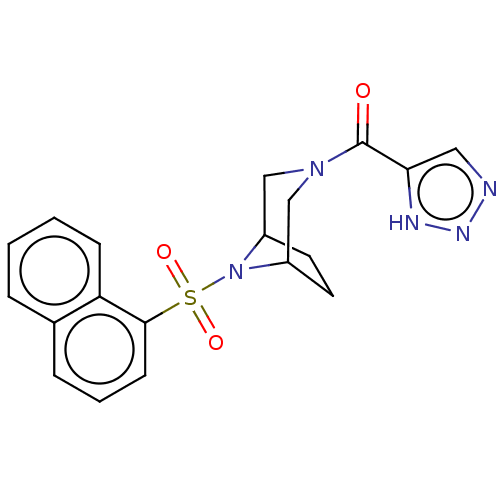 (US10167293, Example 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The enzyme used was recombinant human AKR1C3 (Aldo-keto reductase family 1 member C3; GenBank Accession No. NM_003739). This was expressed in E. coli... | US Patent US10167293 (2019) BindingDB Entry DOI: 10.7270/Q23J3G1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1155 total ) | Next | Last >> |