

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM220118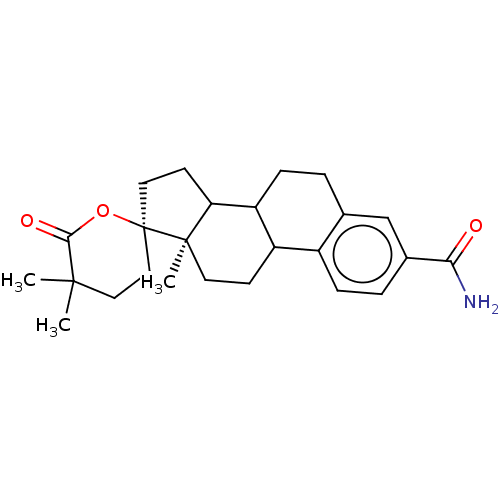 (US9271961, EM1404) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50384947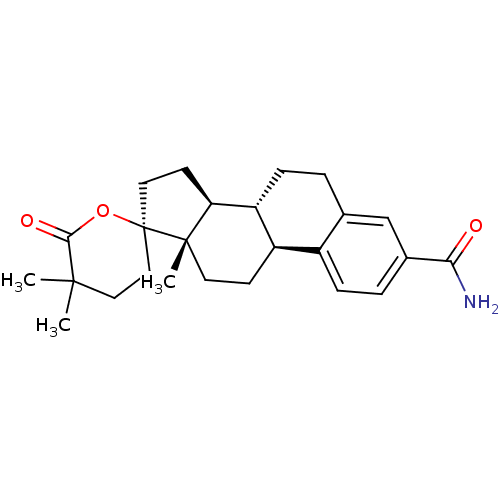 (CHEMBL521703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human GST-tagged 17betaHSD5 expressed in Escherichia coli by radiometric assay | J Nat Prod 75: 716-21 (2012) Article DOI: 10.1021/np201002x BindingDB Entry DOI: 10.7270/Q2Z320P2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM17285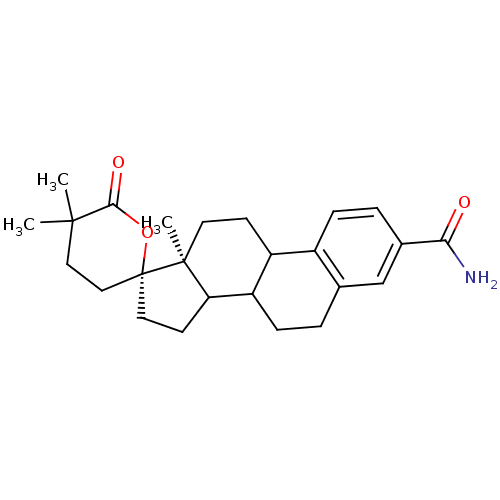 ((2R,15'S)-5,5,15'-trimethyl-6-oxospiro[oxane-2,14'...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.90 | -11.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CHUL | Assay Description A radioactive assay was used for the enzyme kinetics in the presence of EM1404 at different concentrations for its Ki determination. Reactions were i... | J Biol Chem 282: 8368-79 (2007) Article DOI: 10.1074/jbc.M606784200 BindingDB Entry DOI: 10.7270/Q27H1GVB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM35905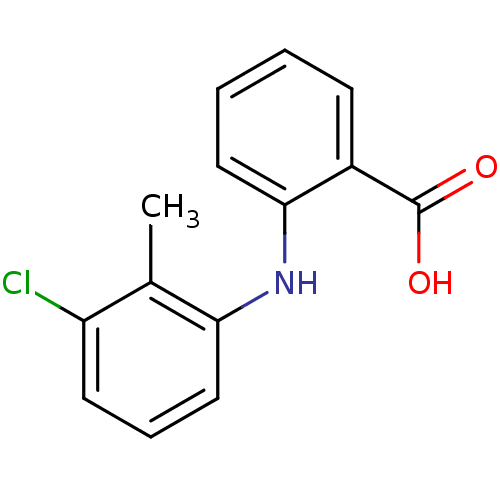 (Tolfenamic acid | cid_610479 | flufenamic acid ana...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AKR1C3 using S-(+)-1,2,3,4-tetrahydro-1-naphthol as substrate | J Nat Prod 75: 716-21 (2012) Article DOI: 10.1021/np201002x BindingDB Entry DOI: 10.7270/Q2Z320P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533570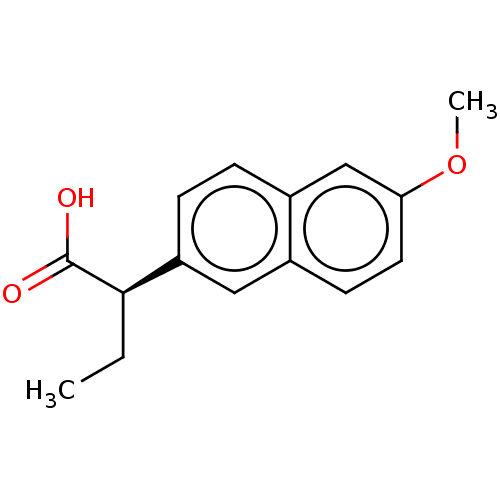 (CHEMBL4435662 | US11459295, Compound LM5750A 8b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preinc... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50384946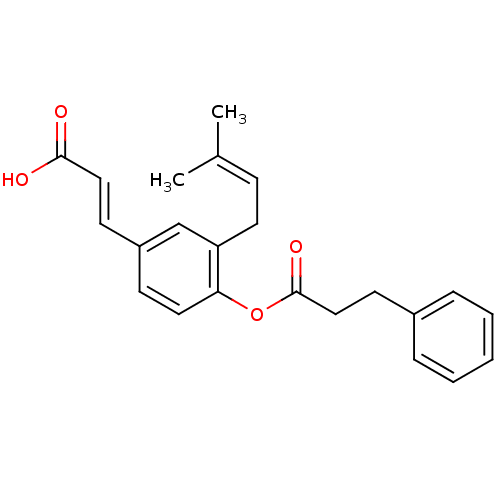 (CHEMBL511708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry | J Nat Prod 75: 716-21 (2012) Article DOI: 10.1021/np201002x BindingDB Entry DOI: 10.7270/Q2Z320P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50384946 (CHEMBL511708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using NADP+ linked S-tetralol as substrate by fluorometr... | J Nat Prod 75: 716-21 (2012) Article DOI: 10.1021/np201002x BindingDB Entry DOI: 10.7270/Q2Z320P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50384946 (CHEMBL511708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry in prese... | J Nat Prod 75: 716-21 (2012) Article DOI: 10.1021/np201002x BindingDB Entry DOI: 10.7270/Q2Z320P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50139171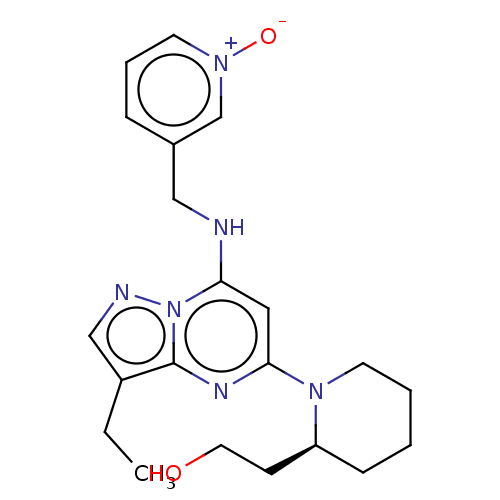 (Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02138 BindingDB Entry DOI: 10.7270/Q23B63QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396749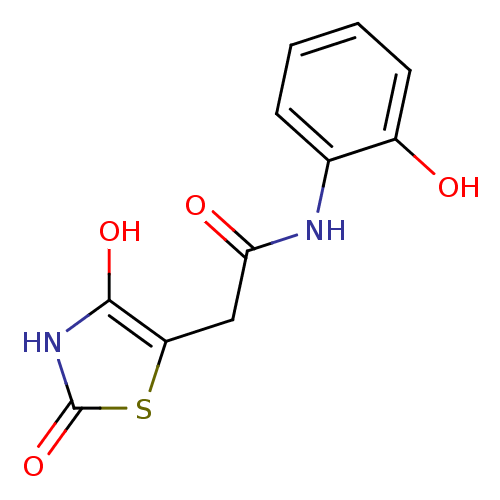 (CHEMBL2172258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 assessed as S-tetralol oxidation by Cheng-Prusoff equation analysis | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM17636 (2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 140 | -9.50 | n/a | n/a | n/a | n/a | n/a | 7.0 | 30 |
The University of Birmingham | Assay Description A fluorescence assay was used to determine the kinetic constants for the oxidation of 3alpha-androstanediol. The fluorescence emission of NADPH at 45... | Cancer Res 64: 1802-10 (2004) Article DOI: 10.1158/0008-5472.can-03-2847 BindingDB Entry DOI: 10.7270/Q2JS9NPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 270 | -9.10 | n/a | n/a | n/a | n/a | n/a | 7.0 | 30 |
The University of Birmingham | Assay Description A fluorescence assay was used to determine the kinetic constants for the oxidation of 3alpha-androstanediol. The fluorescence emission of NADPH at 45... | Cancer Res 64: 1802-10 (2004) Article DOI: 10.1158/0008-5472.can-03-2847 BindingDB Entry DOI: 10.7270/Q2JS9NPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50134036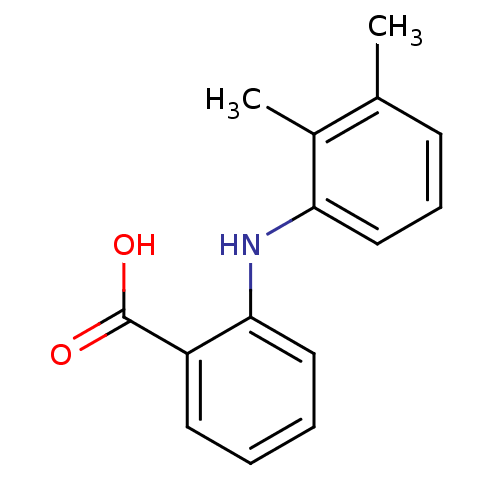 (2-(2,3-Dimethyl-phenylamino)-benzoic acid | 2-(2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM220117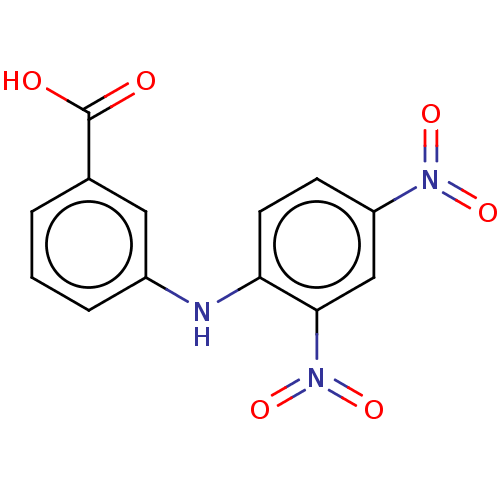 (US9271961, 4-Carboxy-2',4'-dinitrodiphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | US Patent | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50362836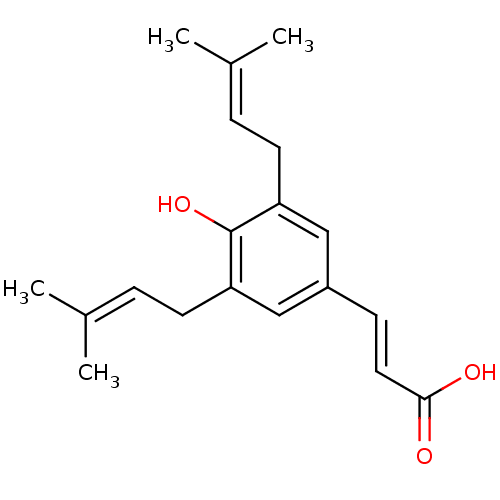 (ARTEPILLIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry in prese... | J Nat Prod 75: 716-21 (2012) Article DOI: 10.1021/np201002x BindingDB Entry DOI: 10.7270/Q2Z320P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50362836 (ARTEPILLIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry | J Nat Prod 75: 716-21 (2012) Article DOI: 10.1021/np201002x BindingDB Entry DOI: 10.7270/Q2Z320P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533570 (CHEMBL4435662 | US11459295, Compound LM5750A 8b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 using assessed as reduction in NADPH-dependent reduction of delat4-androsten-3,17-dione preincubat... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM7585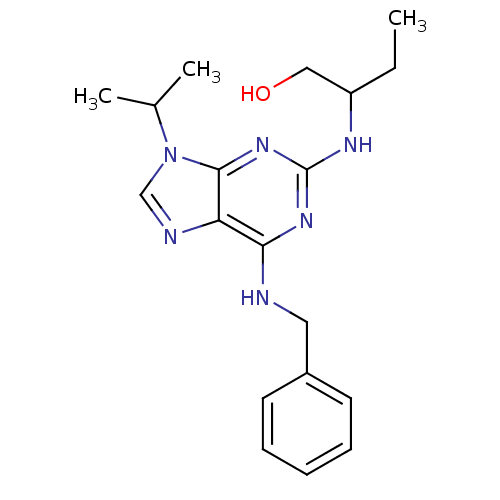 ((R,S)-Roscovitine | 2,6,9-Trisubstituted purine de...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02138 BindingDB Entry DOI: 10.7270/Q23B63QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM220121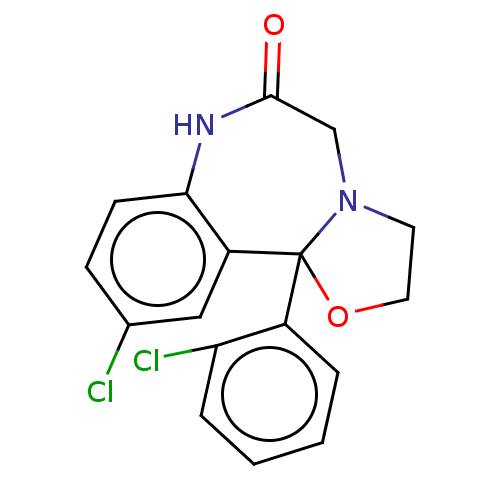 (US9271961, Cloxazolam) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid UniChem | US Patent | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396749 (CHEMBL2172258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.10E+3 | -7.87 | n/a | n/a | n/a | n/a | n/a | 7.0 | 30 |
The University of Birmingham | Assay Description Enzyme activity was measured in the reductive direction against varying concentrations of androsterone. Initial velocities were measured by observing... | Cancer Res 64: 1802-10 (2004) Article DOI: 10.1158/0008-5472.can-03-2847 BindingDB Entry DOI: 10.7270/Q2JS9NPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396748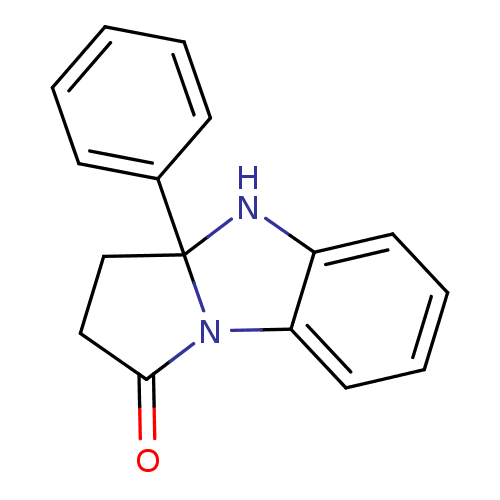 (CHEMBL366350) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 assessed as S-tetralol oxidation by Cheng-Prusoff equation analysis | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM17636 (2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.10E+3 | -7.64 | n/a | n/a | n/a | n/a | n/a | 7.0 | 30 |
The University of Birmingham | Assay Description Enzyme activity was measured in the reductive direction against varying concentrations of androsterone. Initial velocities were measured by observing... | Cancer Res 64: 1802-10 (2004) Article DOI: 10.1158/0008-5472.can-03-2847 BindingDB Entry DOI: 10.7270/Q2JS9NPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396731 (CHEMBL2172254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50219490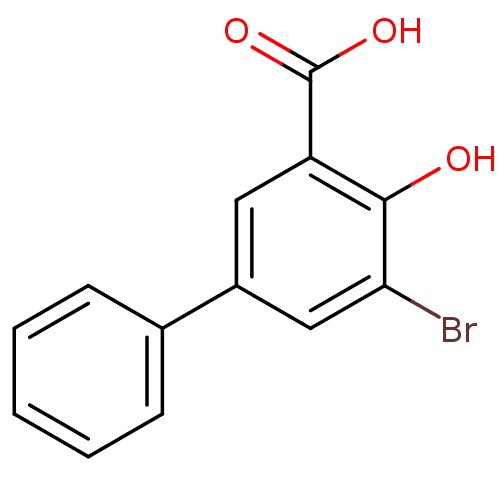 (3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human recombinant type 2 3-alpha-HSD expressed in Escherichia coli JM109 | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50546239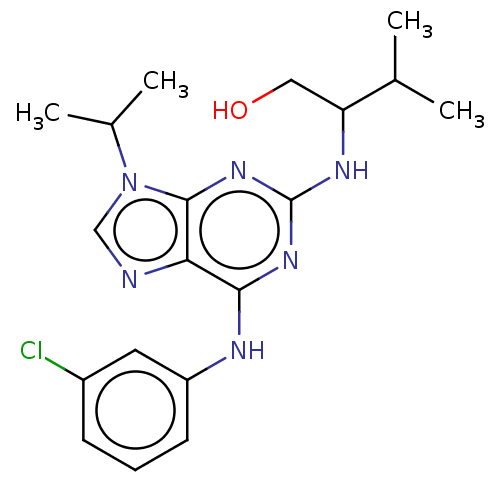 (CHEMBL103579) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02138 BindingDB Entry DOI: 10.7270/Q23B63QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM220116 (US9271961, CBM) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396739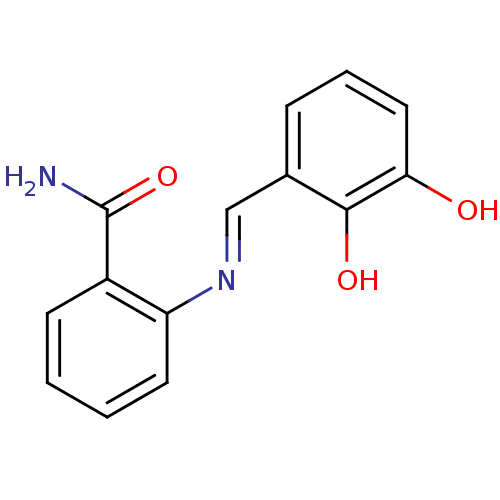 (CHEMBL2172243) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50380363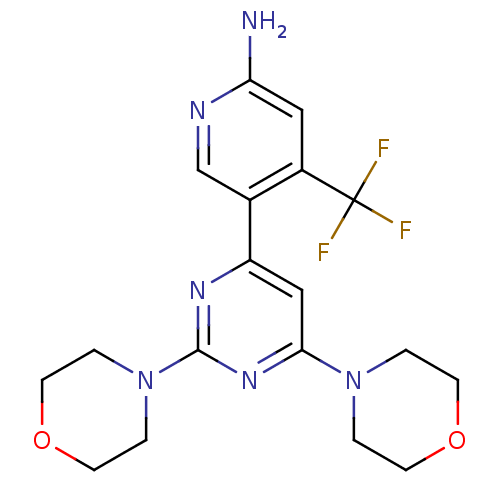 (CHEMBL2017974 | US10173995, Compound 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC method based Linewea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02138 BindingDB Entry DOI: 10.7270/Q23B63QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50293439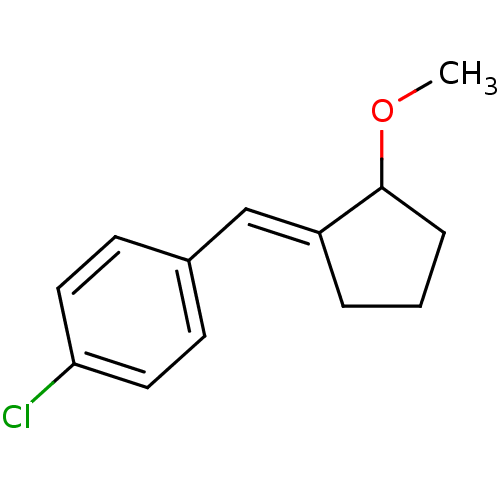 (2-(4-chlorobenzylidene)cyclopentylmethyl ether | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-tagged AKR1C3 expressed in Escherichia coli BL21 | Eur J Med Chem 44: 2563-71 (2009) Article DOI: 10.1016/j.ejmech.2009.01.028 BindingDB Entry DOI: 10.7270/Q2319VW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50293436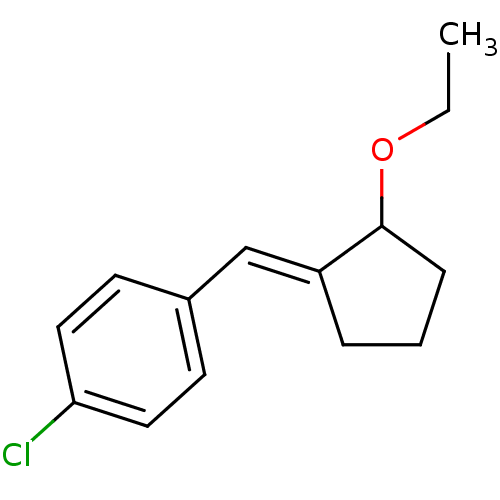 (2-(4-chlorobenzylidene)cyclopentyl ethyl ether | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-tagged AKR1C3 expressed in Escherichia coli BL21 | Eur J Med Chem 44: 2563-71 (2009) Article DOI: 10.1016/j.ejmech.2009.01.028 BindingDB Entry DOI: 10.7270/Q2319VW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396691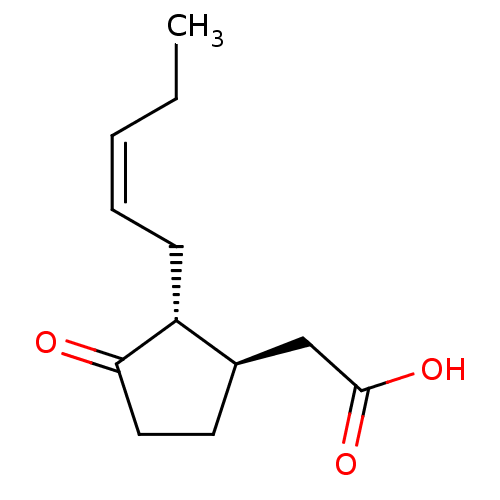 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | US Patent | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50240769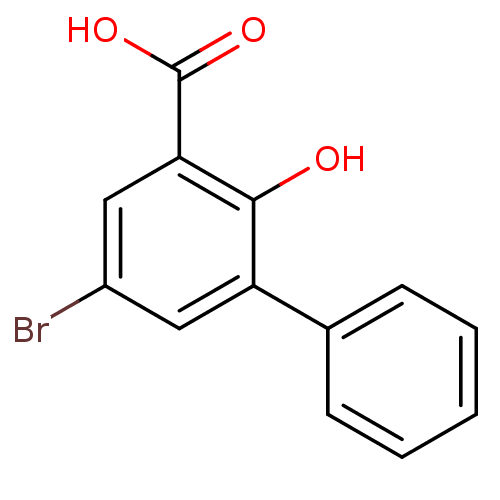 (3-Phenyl-5-bromosalicylic acid | 5-Bromo-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human recombinant type 2 3-alpha-HSD expressed in Escherichia coli JM109 | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50249792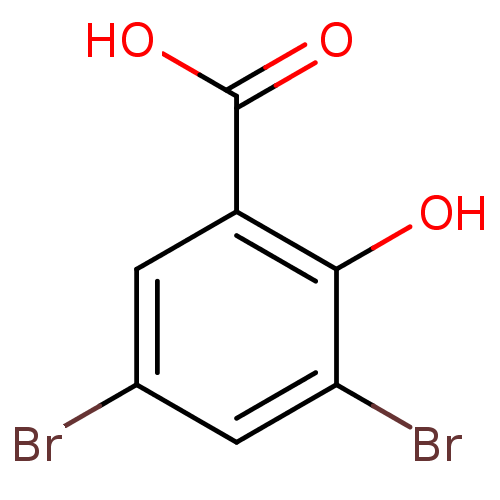 (3,5-Dibromosalicylic acid | CHEMBL447448) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human recombinant type 2 3-alpha-HSD expressed in Escherichia coli JM109 | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Tech University Health Sciences Center Curated by ChEMBL | Assay Description Inhibition of recombinant full-length human N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21 (DE3) using phenanthrenequinone as subst... | J Med Chem 62: 3590-3616 (2019) Article DOI: 10.1021/acs.jmedchem.9b00090 BindingDB Entry DOI: 10.7270/Q2QF8X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396748 (CHEMBL366350) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50293435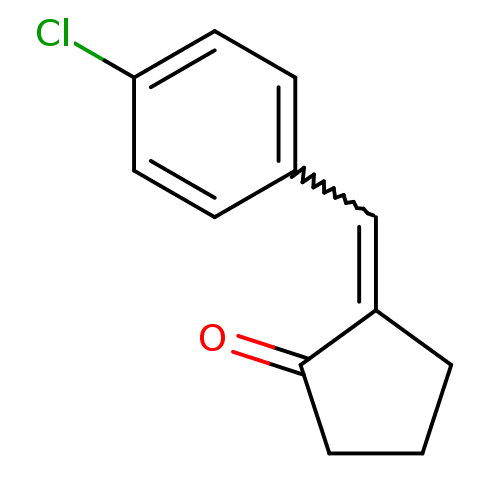 (2-(4-Chlorobenzylidene)cyclopentanone | CHEMBL5623...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-tagged AKR1C3 expressed in Escherichia coli BL21 | Eur J Med Chem 44: 2563-71 (2009) Article DOI: 10.1016/j.ejmech.2009.01.028 BindingDB Entry DOI: 10.7270/Q2319VW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396740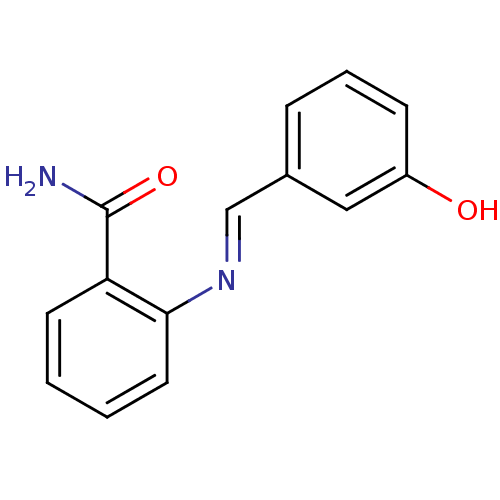 (CHEMBL2172242) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM34643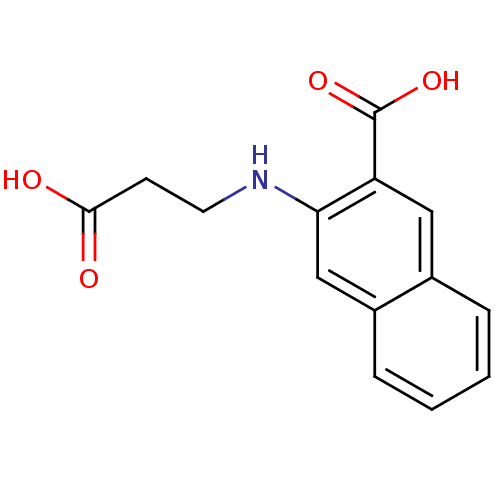 (3-(2-carboxyethylamino)-2-naphthalenecarboxylic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396732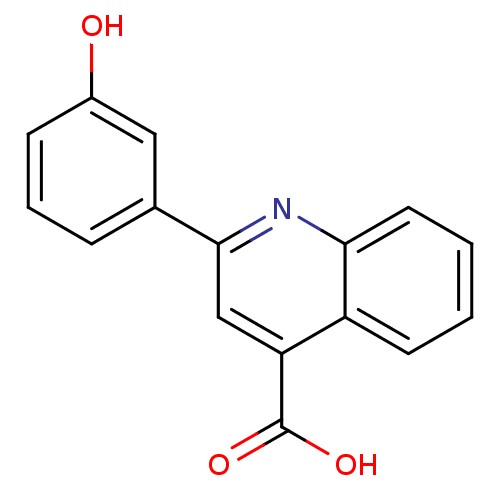 (CHEMBL2172252) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50293437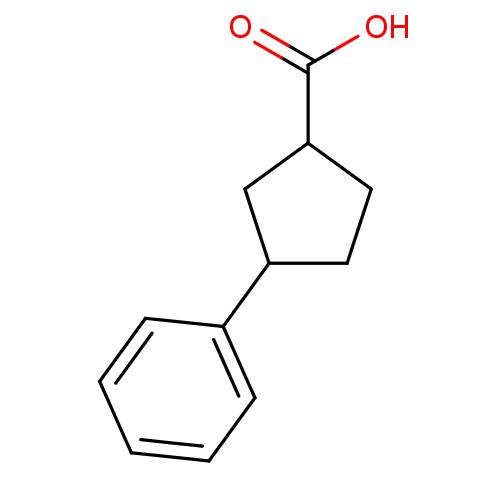 (3-Phenylcyclopentanecarboxylic acid | CHEMBL554283) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-tagged AKR1C3 expressed in Escherichia coli BL21 | Eur J Med Chem 44: 2563-71 (2009) Article DOI: 10.1016/j.ejmech.2009.01.028 BindingDB Entry DOI: 10.7270/Q2319VW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396736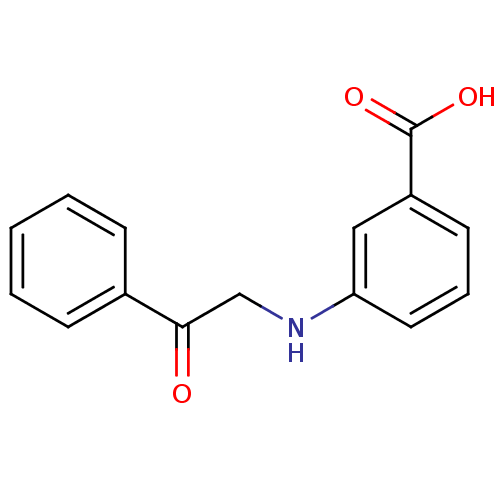 (CHEMBL2172249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396729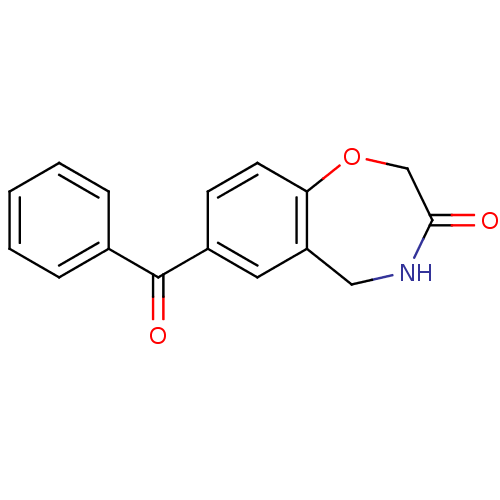 (CHEMBL1414132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396741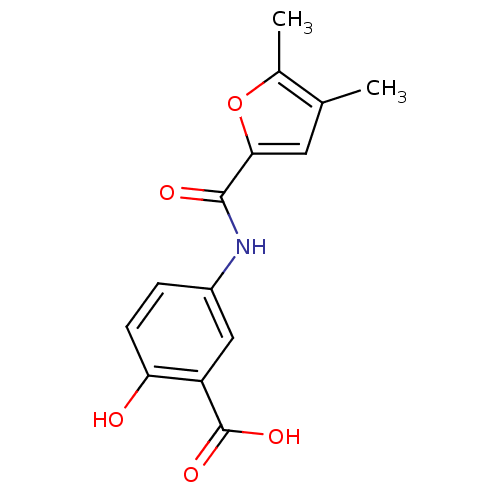 (CHEMBL2172240) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396745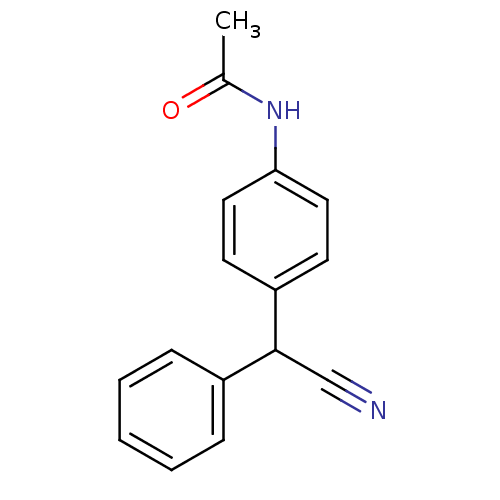 (CHEMBL1580175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM26269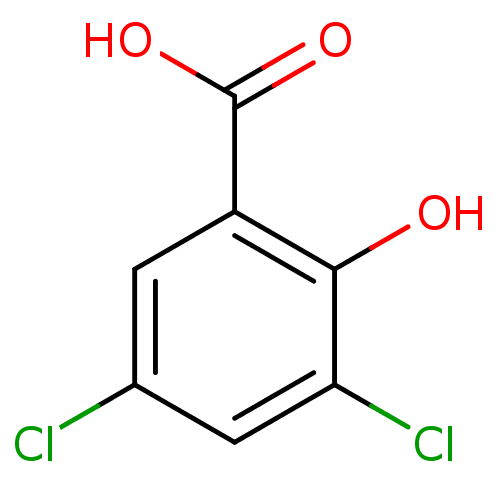 (3,5-dichloro-2-hydroxybenzoic acid | 3,5-dichloros...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.40E+4 | -5.49 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Monash University | Assay Description The activity was assayed by measuring the rate of change in NADPH fluorescence (at 455 nm with an excitation wavelength of 340 nm) at 298 K. When the... | J Med Chem 51: 4844-8 (2008) Article DOI: 10.1021/jm8003575 BindingDB Entry DOI: 10.7270/Q2MG7MTW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396735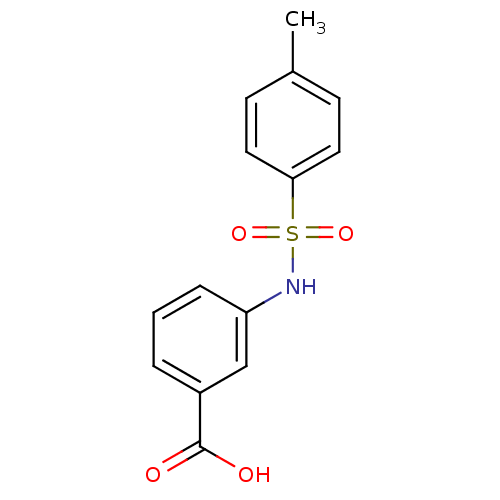 (CHEMBL2172250) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396733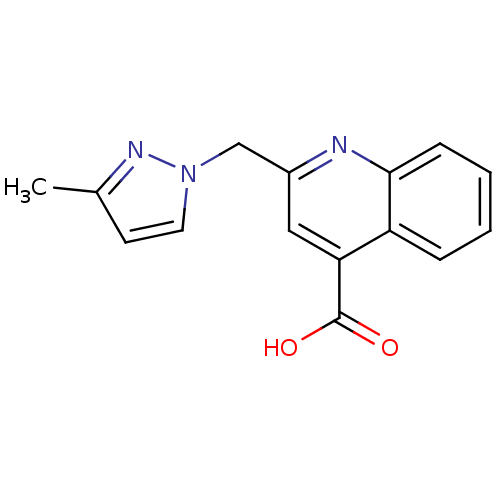 (CHEMBL2172251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396750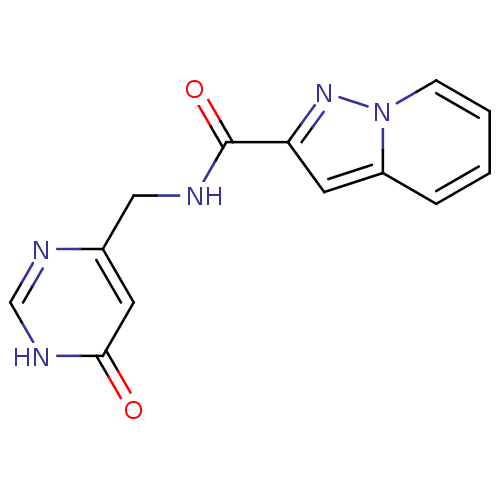 (CHEMBL2172259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |