 Found 167 hits of kd for UniProtKB: Q06187
Found 167 hits of kd for UniProtKB: Q06187 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36516
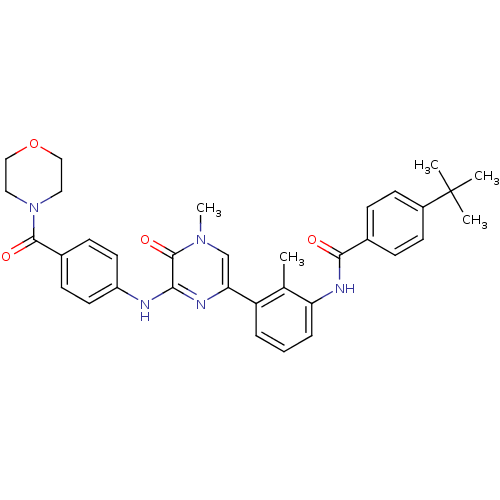
(4-(tert-Butyl)-N-(2-methyl-3-(4-methyl-6-((4-(morp...)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C34H37N5O4/c1-22-27(7-6-8-28(22)37-31(40)23-9-13-25(14-10-23)34(2,3)4)29-21-38(5)33(42)30(36-29)35-26-15-11-24(12-16-26)32(41)39-17-19-43-20-18-39/h6-16,21H,17-20H2,1-5H3,(H,35,36)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | 1.5 | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals
| Assay Description
Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. |
Nat Chem Biol 7: 41-50 (2011)
Article DOI: 10.1038/nchembio.481
BindingDB Entry DOI: 10.7270/Q2B56H2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM4779
(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2BZ64F4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PCBioAssay
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2BZ64F4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM31090
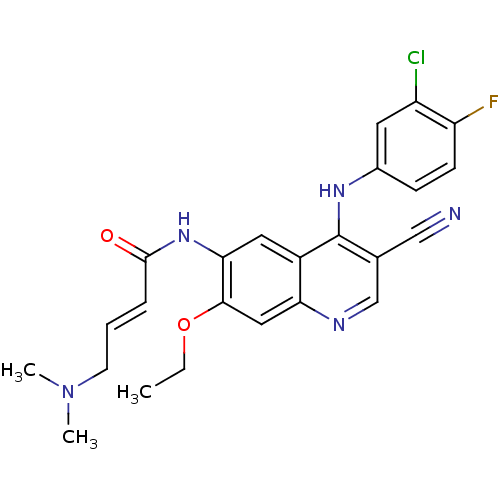
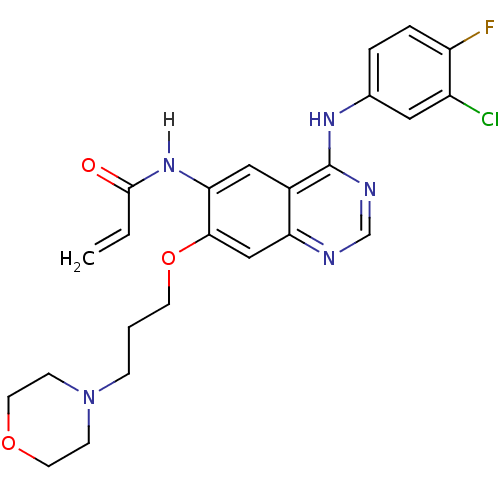
((E)-N-[4-(3-chloro-4-fluoro-anilino)-3-cyano-7-eth...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C24H23ClFN5O2/c1-4-33-22-12-20-17(11-21(22)30-23(32)6-5-9-31(2)3)24(15(13-27)14-28-20)29-16-7-8-19(26)18(25)10-16/h5-8,10-12,14H,4,9H2,1-3H3,(H,28,29)(H,30,32)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2BZ64F4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM31093
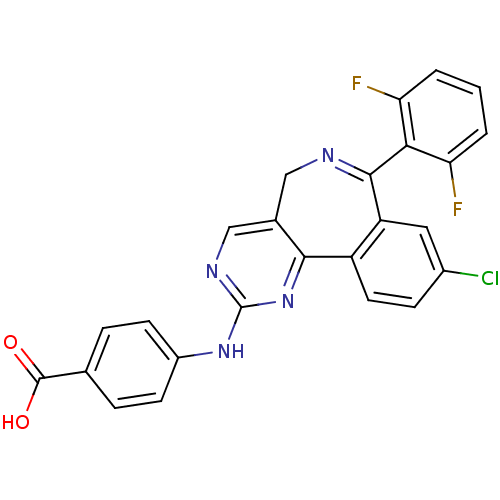
(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2BZ64F4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM31096
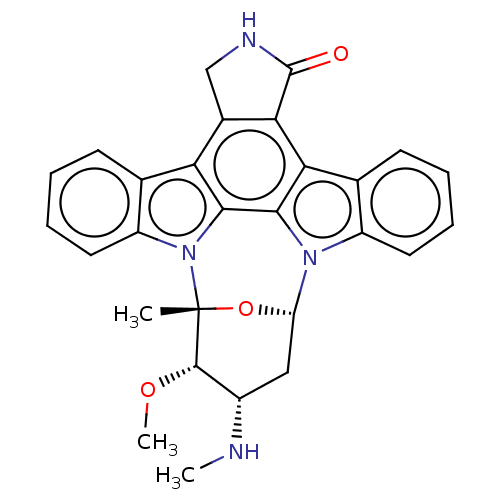
(CHEMBL290084 | Staurosporine | cid_451705)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2BZ64F4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2BZ64F4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM76963
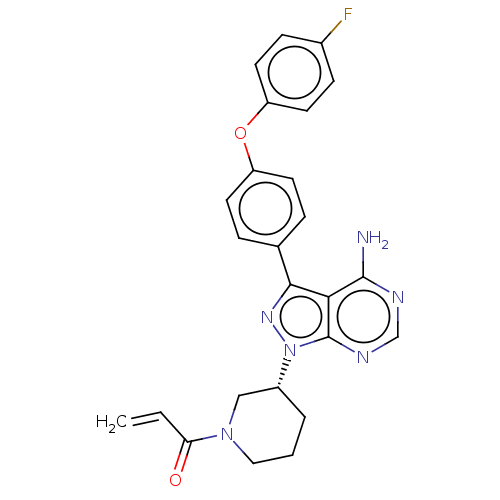
(US9694011, Example 1 (Compound 17))Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccc(F)cc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C25H23FN6O2/c1-2-21(33)31-13-3-4-18(14-31)32-25-22(24(27)28-15-29-25)23(30-32)16-5-9-19(10-6-16)34-20-11-7-17(26)8-12-20/h2,5-12,15,18H,1,3-4,13-14H2,(H2,27,28,29)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 32 |
Jiangsu Medolution Ltd
US Patent
| Assay Description
Kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phase and infected with T7 ph... |
US Patent US9694011 (2017)
BindingDB Entry DOI: 10.7270/Q20K26Q4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM76973
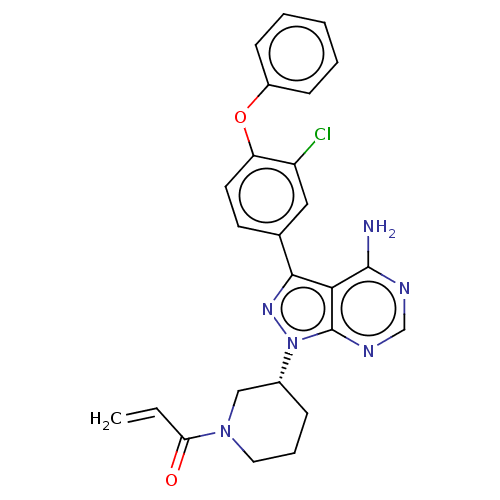
(US9694011, Example 2 (Compound 18))Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)c(Cl)c3)c12)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C25H23ClN6O2/c1-2-21(33)31-12-6-7-17(14-31)32-25-22(24(27)28-15-29-25)23(30-32)16-10-11-20(19(26)13-16)34-18-8-4-3-5-9-18/h2-5,8-11,13,15,17H,1,6-7,12,14H2,(H2,27,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 32 |
Jiangsu Medolution Ltd
US Patent
| Assay Description
Kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phase and infected with T7 ph... |
US Patent US9694011 (2017)
BindingDB Entry DOI: 10.7270/Q20K26Q4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for BTK; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to BTK |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50308060
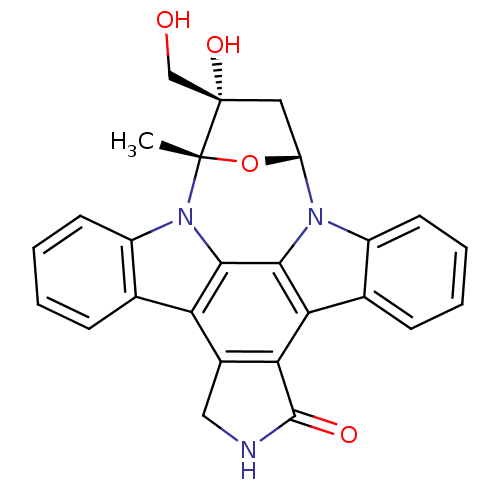
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to BTK |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50026612
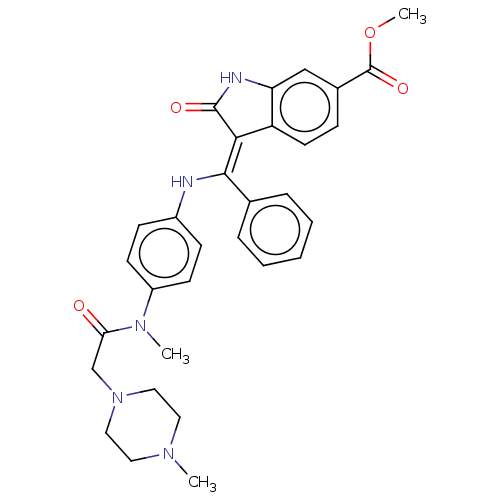
(BIBF-1120 | Nintedanib | US10981896, Compound Nint...)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-14-9-22(31(39)40-3)19-26(25)33-30(28)38/h4-14,19,32H,15-18,20H2,1-3H3,(H,33,38)/b29-28- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for full-length BTK |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for full-length BTK |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50131843
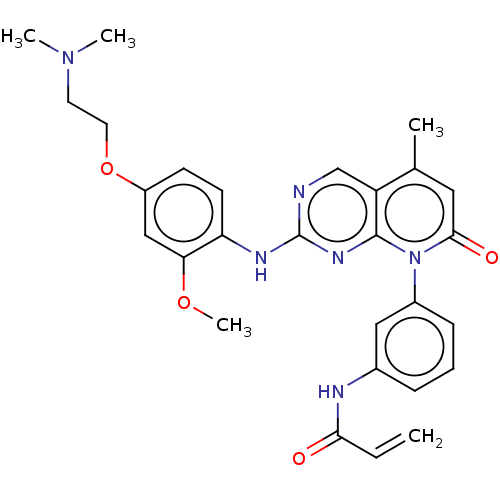
(CHEMBL3633152)Show SMILES COc1cc(OCCN(C)C)ccc1Nc1ncc2c(C)cc(=O)n(-c3cccc(NC(=O)C=C)c3)c2n1 Show InChI InChI=1S/C28H30N6O4/c1-6-25(35)30-19-8-7-9-20(15-19)34-26(36)14-18(2)22-17-29-28(32-27(22)34)31-23-11-10-21(16-24(23)37-5)38-13-12-33(3)4/h6-11,14-17H,1,12-13H2,2-5H3,(H,30,35)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against delta opioid receptor in mouse hot plate test |
ACS Med Chem Lett 6: 987-92 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00193
BindingDB Entry DOI: 10.7270/Q21C1ZPN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068541
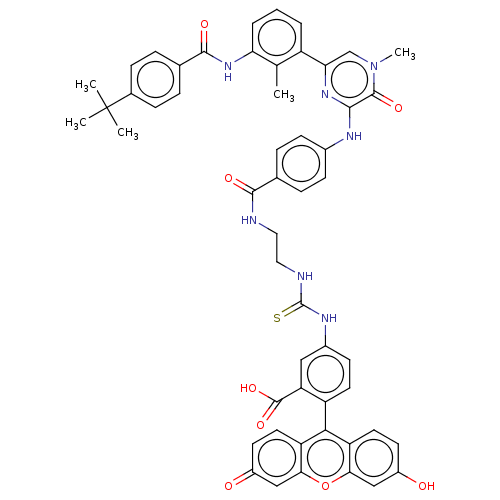
(CHEMBL3400825)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)NCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(14.47,-12.78,;13.4,-12.18,;13.37,-10.64,;14.7,-9.85,;14.67,-8.31,;13.59,-7.71,;15.99,-7.51,;15.97,-5.97,;17.29,-5.18,;18.63,-5.93,;18.66,-7.47,;17.34,-8.26,;19.96,-5.14,;19.94,-3.91,;21.03,-5.74,;21.01,-4.51,;12.03,-9.89,;10.71,-10.68,;10.73,-12.22,;12.08,-12.97,;12.1,-14.51,;13.44,-15.26,;13.46,-16.8,;14.54,-17.41,;12.14,-17.59,;12.16,-18.82,;10.8,-16.84,;9.47,-17.63,;8.13,-16.87,;6.8,-17.66,;5.46,-16.9,;5.44,-15.36,;6.77,-14.58,;8.11,-15.33,;4.1,-14.6,;3.04,-15.23,;4.09,-13.06,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;10.78,-15.3,)| Show InChI InChI=1S/C53H47N7O8S/c1-29-37(7-6-8-42(29)59-49(64)31-9-13-32(14-10-31)53(2,3)4)43-28-60(5)50(65)47(58-43)56-33-15-11-30(12-16-33)48(63)54-23-24-55-52(69)57-34-17-20-38(41(25-34)51(66)67)46-39-21-18-35(61)26-44(39)68-45-27-36(62)19-22-40(45)46/h6-22,25-28,61H,23-24H2,1-5H3,(H,54,63)(H,56,58)(H,59,64)(H,66,67)(H2,55,57,69) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068542
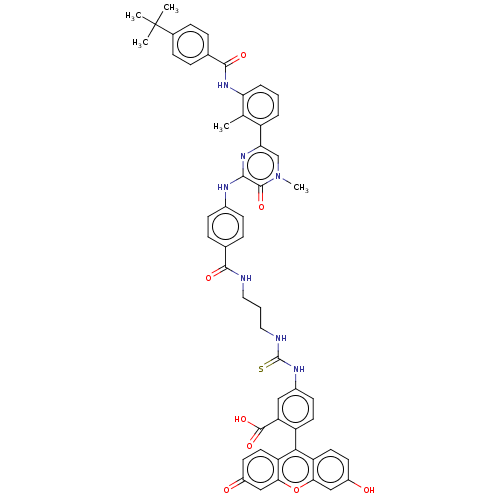
(CHEMBL3400826)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)NCCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(-1.18,-23.55,;-1.18,-22.32,;-2.52,-21.55,;-3.85,-22.32,;-5.18,-21.55,;-5.18,-20.32,;-6.52,-22.32,;-7.85,-21.55,;-9.19,-22.32,;-9.18,-23.86,;-7.85,-24.63,;-6.52,-23.86,;-10.52,-24.64,;-11.59,-24.02,;-10.51,-25.87,;-11.58,-25.26,;-2.52,-20.01,;-1.19,-19.24,;.15,-20,;.15,-21.54,;1.49,-22.31,;1.49,-23.85,;2.83,-24.61,;2.83,-25.85,;4.16,-23.84,;5.23,-24.45,;4.16,-22.3,;5.49,-21.52,;5.48,-19.98,;6.81,-19.2,;6.8,-17.66,;5.46,-16.9,;4.13,-17.68,;4.14,-19.22,;5.44,-15.36,;6.51,-14.74,;4.1,-14.6,;4.09,-13.06,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;2.82,-21.54,)| Show InChI InChI=1S/C54H49N7O8S/c1-30-38(8-6-9-43(30)60-50(65)32-10-14-33(15-11-32)54(2,3)4)44-29-61(5)51(66)48(59-44)57-34-16-12-31(13-17-34)49(64)55-24-7-25-56-53(70)58-35-18-21-39(42(26-35)52(67)68)47-40-22-19-36(62)27-45(40)69-46-28-37(63)20-23-41(46)47/h6,8-23,26-29,62H,7,24-25H2,1-5H3,(H,55,64)(H,57,59)(H,60,65)(H,67,68)(H2,56,58,70) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068543
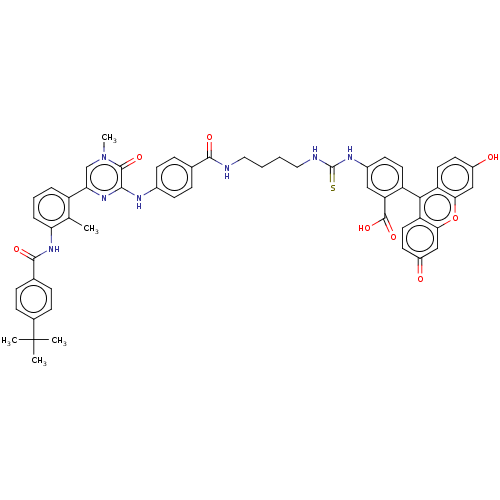
(CHEMBL3402353)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)NCCCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(15.83,-15.08,;14.75,-14.48,;14.73,-12.94,;16.05,-12.15,;16.02,-10.61,;14.95,-10.01,;17.35,-9.81,;17.32,-8.27,;18.64,-7.48,;19.99,-8.23,;20.01,-9.77,;18.69,-10.56,;21.31,-7.44,;21.29,-6.21,;22.39,-8.04,;22.37,-6.81,;13.38,-12.19,;12.06,-12.98,;12.09,-14.52,;13.43,-15.27,;13.45,-16.81,;14.8,-17.56,;14.82,-19.1,;15.89,-19.71,;13.5,-19.89,;13.51,-21.12,;12.15,-19.14,;10.83,-19.93,;9.48,-19.17,;8.16,-19.96,;6.81,-19.2,;6.8,-17.66,;8.12,-16.88,;9.47,-17.63,;5.46,-16.9,;4.4,-17.53,;5.44,-15.36,;4.1,-14.6,;4.09,-13.06,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;12.13,-17.6,)| Show InChI InChI=1S/C55H51N7O8S/c1-31-39(9-8-10-44(31)61-51(66)33-11-15-34(16-12-33)55(2,3)4)45-30-62(5)52(67)49(60-45)58-35-17-13-32(14-18-35)50(65)56-25-6-7-26-57-54(71)59-36-19-22-40(43(27-36)53(68)69)48-41-23-20-37(63)28-46(41)70-47-29-38(64)21-24-42(47)48/h8-24,27-30,63H,6-7,25-26H2,1-5H3,(H,56,65)(H,58,60)(H,61,66)(H,68,69)(H2,57,59,71) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50068544
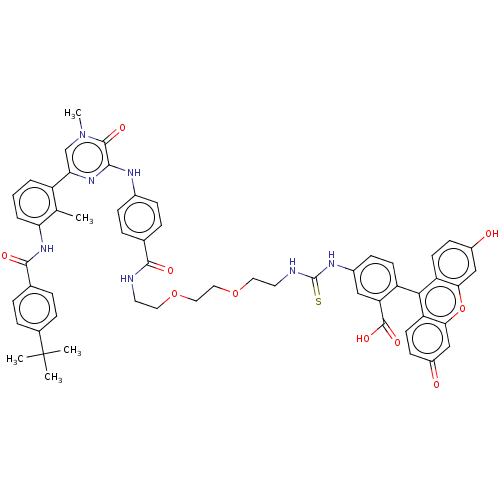
(CHEMBL3402354)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)NCCOCCOCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(18.54,-19.68,;17.46,-19.08,;17.44,-17.54,;18.76,-16.75,;18.73,-15.21,;17.66,-14.61,;20.05,-14.41,;20.03,-12.87,;21.35,-12.08,;22.7,-12.83,;22.72,-14.37,;21.4,-15.16,;24.02,-12.04,;24,-10.81,;25.1,-12.64,;25.08,-11.41,;16.09,-16.79,;14.77,-17.58,;14.8,-19.12,;16.14,-19.87,;16.16,-21.41,;17.51,-22.16,;17.53,-23.7,;18.6,-24.31,;16.2,-24.49,;16.22,-25.72,;14.86,-23.74,;13.53,-24.53,;12.19,-23.77,;10.87,-24.56,;9.52,-23.8,;9.51,-22.26,;10.83,-21.48,;12.17,-22.23,;8.17,-21.5,;7.11,-22.13,;8.15,-19.96,;6.81,-19.2,;6.8,-17.66,;5.46,-16.9,;5.44,-15.36,;4.1,-14.6,;4.09,-13.06,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;14.84,-22.2,)| Show InChI InChI=1S/C57H55N7O10S/c1-33-41(7-6-8-46(33)63-53(68)35-9-13-36(14-10-35)57(2,3)4)47-32-64(5)54(69)51(62-47)60-37-15-11-34(12-16-37)52(67)58-23-25-72-27-28-73-26-24-59-56(75)61-38-17-20-42(45(29-38)55(70)71)50-43-21-18-39(65)30-48(43)74-49-31-40(66)19-22-44(49)50/h6-22,29-32,65H,23-28H2,1-5H3,(H,58,67)(H,60,62)(H,63,68)(H,70,71)(H2,59,61,75) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
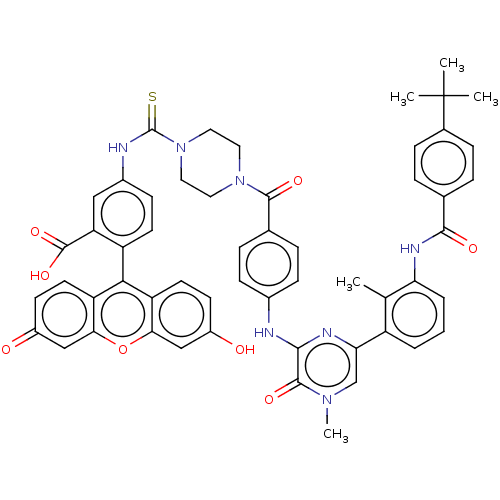
(Homo sapiens (Human)) | BDBM50068574
(CHEMBL3402355)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCN(CC2)C(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)n1 |(-8.97,-12.95,;-7.9,-12.33,;-7.9,-10.79,;-9.23,-10.02,;-9.23,-8.47,;-8.16,-7.86,;-10.56,-7.7,;-10.56,-6.16,;-11.89,-5.39,;-13.23,-6.16,;-13.23,-7.7,;-11.9,-8.47,;-14.56,-5.38,;-14.56,-4.15,;-15.63,-6,;-15.62,-4.77,;-6.57,-10.02,;-5.23,-10.8,;-5.24,-12.34,;-6.57,-13.1,;-6.57,-14.64,;-7.91,-15.41,;-7.91,-16.95,;-8.97,-17.57,;-6.57,-17.72,;-6.57,-18.96,;-5.24,-16.95,;-3.9,-17.72,;-2.57,-16.95,;-1.23,-17.71,;.1,-16.94,;.09,-15.4,;-1.24,-14.63,;-2.57,-15.41,;1.42,-14.62,;2.49,-15.23,;1.42,-13.08,;2.74,-12.3,;2.73,-10.76,;1.39,-10,;.07,-10.78,;.08,-12.32,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;-5.24,-15.41,)| Show InChI InChI=1S/C55H49N7O8S/c1-31-39(7-6-8-44(31)59-50(65)32-9-13-34(14-10-32)55(2,3)4)45-30-60(5)52(67)49(58-45)56-35-15-11-33(12-16-35)51(66)61-23-25-62(26-24-61)54(71)57-36-17-20-40(43(27-36)53(68)69)48-41-21-18-37(63)28-46(41)70-47-29-38(64)19-22-42(47)48/h6-22,27-30,63H,23-26H2,1-5H3,(H,56,58)(H,57,71)(H,59,65)(H,68,69) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
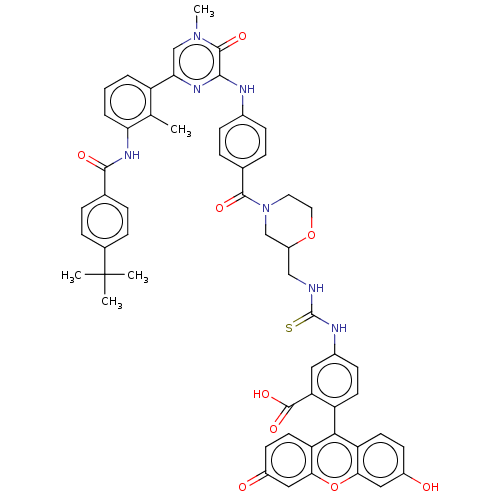
(Homo sapiens (Human)) | BDBM50068595
(CHEMBL3402356)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOC(CNC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccc(O)cc4oc4cc(=O)ccc34)C2)n1 |(-1.21,-23.53,;-1.21,-22.3,;-2.54,-21.53,;-3.88,-22.29,;-5.21,-21.52,;-5.21,-20.29,;-6.55,-22.28,;-7.88,-21.51,;-9.22,-22.28,;-9.22,-23.82,;-7.89,-24.59,;-6.55,-23.82,;-10.56,-24.59,;-11.62,-23.97,;-10.56,-25.82,;-11.62,-25.2,;-2.54,-19.99,;-1.21,-19.22,;.13,-19.99,;.12,-21.53,;1.46,-22.3,;1.46,-23.84,;2.79,-24.61,;2.79,-25.84,;4.12,-23.84,;5.19,-24.46,;4.12,-22.3,;5.46,-21.53,;5.46,-19.99,;6.79,-19.21,;6.78,-17.67,;5.44,-16.91,;4.11,-17.68,;4.12,-19.22,;5.44,-15.37,;6.5,-14.75,;4.1,-14.6,;2.77,-15.38,;1.43,-14.62,;1.42,-13.08,;2.75,-12.3,;2.74,-10.76,;1.39,-10,;1.38,-8.46,;2.44,-7.84,;.04,-7.7,;.03,-6.16,;1.35,-5.38,;1.34,-3.84,;0,-3.08,;-1.32,-3.86,;-1.31,-5.4,;-2.66,-3.1,;-2.67,-2.07,;-3.73,-3.73,;,-1.54,;1.31,-.77,;2.67,-1.54,;4,-.77,;4,.77,;5.07,1.39,;2.67,1.54,;1.31,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.07,1.39,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;4.09,-13.06,;2.79,-21.53,)| Show InChI InChI=1S/C56H51N7O9S/c1-31-40(7-6-8-45(31)61-51(66)32-9-13-34(14-10-32)56(2,3)4)46-30-62(5)53(68)50(60-46)58-35-15-11-33(12-16-35)52(67)63-23-24-71-39(29-63)28-57-55(73)59-36-17-20-41(44(25-36)54(69)70)49-42-21-18-37(64)26-47(42)72-48-27-38(65)19-22-43(48)49/h6-22,25-27,30,39,64H,23-24,28-29H2,1-5H3,(H,58,60)(H,61,66)(H,69,70)(H2,57,59,73) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated and unactivated BTK (unknown origin) incubated for 1 hr by TR-FRET assay |
Bioorg Med Chem Lett 25: 2033-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.001
BindingDB Entry DOI: 10.7270/Q2VX0J60 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for BTK; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50300690
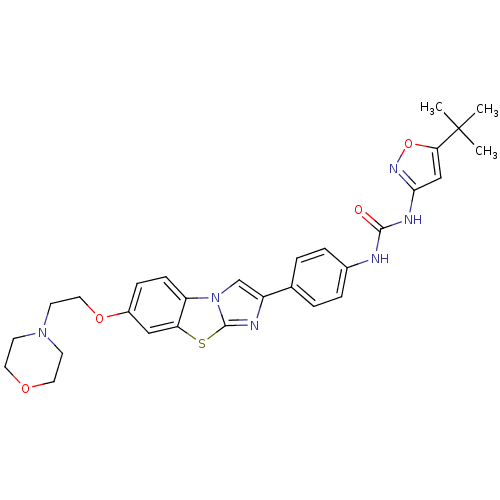
(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to BTK |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to BTK |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13535
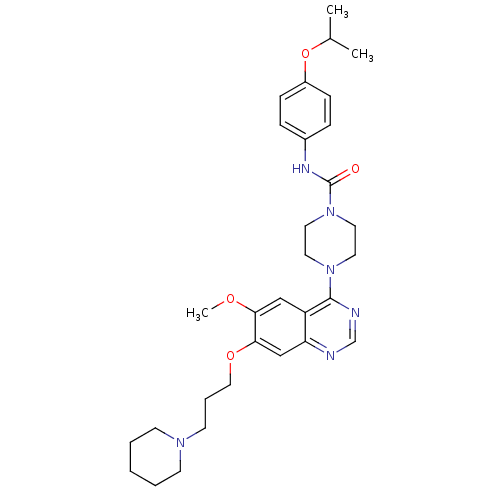
(4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin...)Show SMILES COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-23(2)41-25-10-8-24(9-11-25)34-31(38)37-17-15-36(16-18-37)30-26-20-28(39-3)29(21-27(26)32-22-33-30)40-19-7-14-35-12-5-4-6-13-35/h8-11,20-23H,4-7,12-19H2,1-3H3,(H,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to BTK |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50326054
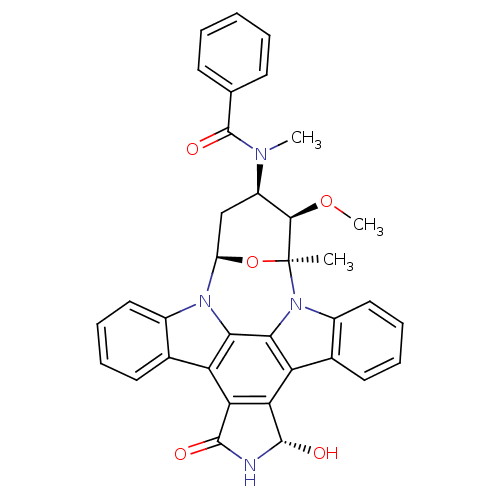
(CHEMBL1240703)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4[C@H](O)NC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O5/c1-35-31(43-3)23(37(2)34(42)18-11-5-4-6-12-18)17-24(44-35)38-21-15-9-7-13-19(21)25-27-28(33(41)36-32(27)40)26-20-14-8-10-16-22(20)39(35)30(26)29(25)38/h4-16,23-24,31,33,41H,17H2,1-3H3,(H,36,40)/t23-,24-,31-,33+,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to BTK |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM4814
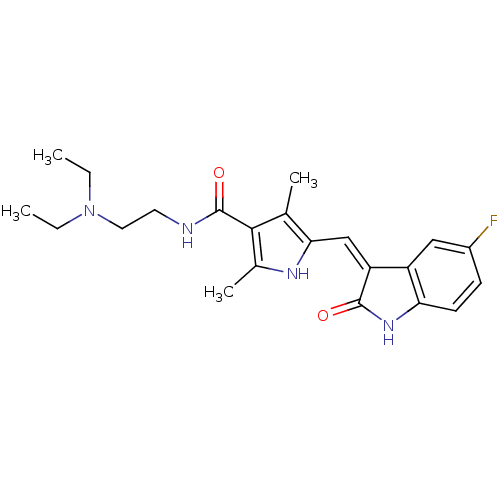
(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to BTK |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50309910
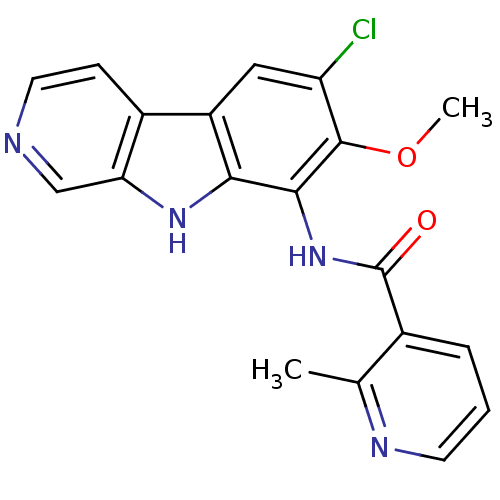
(CHEMBL608154 | ML-120B | N-(6-chloro-7-methoxy-9H-...)Show SMILES COc1c(Cl)cc2c3ccncc3[nH]c2c1NC(=O)c1cccnc1C Show InChI InChI=1S/C19H15ClN4O2/c1-10-11(4-3-6-22-10)19(25)24-17-16-13(8-14(20)18(17)26-2)12-5-7-21-9-15(12)23-16/h3-9,23H,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50355500
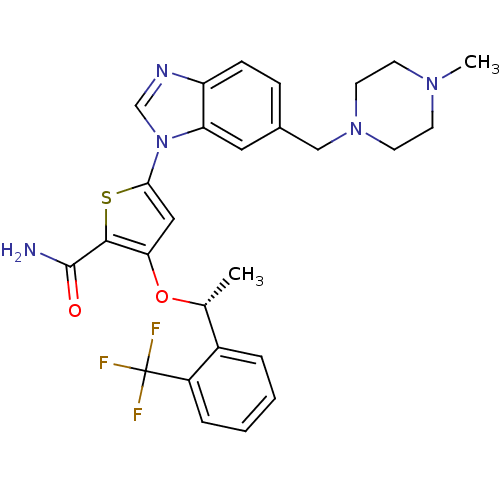
(CHEMBL1908394 | US9695172, GSK461364)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(CN3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N5O2S/c1-17(19-5-3-4-6-20(19)27(28,29)30)37-23-14-24(38-25(23)26(31)36)35-16-32-21-8-7-18(13-22(21)35)15-34-11-9-33(2)10-12-34/h3-8,13-14,16-17H,9-12,15H2,1-2H3,(H2,31,36)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13535

(4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin...)Show SMILES COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-23(2)41-25-10-8-24(9-11-25)34-31(38)37-17-15-36(16-18-37)30-26-20-28(39-3)29(21-27(26)32-22-33-30)40-19-7-14-35-12-5-4-6-13-35/h8-11,20-23H,4-7,12-19H2,1-3H3,(H,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM26474
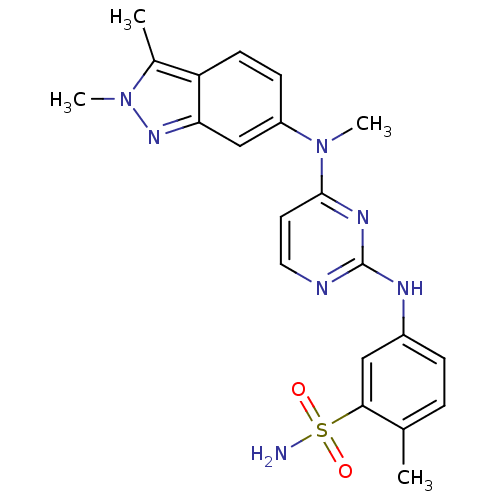
(5-({4-[(2,3-dimethyl-2H-indazol-6-yl)(methyl)amino...)Show SMILES CN(c1ccc2c(C)n(C)nc2c1)c1ccnc(Nc2ccc(C)c(c2)S(N)(=O)=O)n1 Show InChI InChI=1S/C21H23N7O2S/c1-13-5-6-15(11-19(13)31(22,29)30)24-21-23-10-9-20(25-21)27(3)16-7-8-17-14(2)28(4)26-18(17)12-16/h5-12H,1-4H3,(H2,22,29,30)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM25919
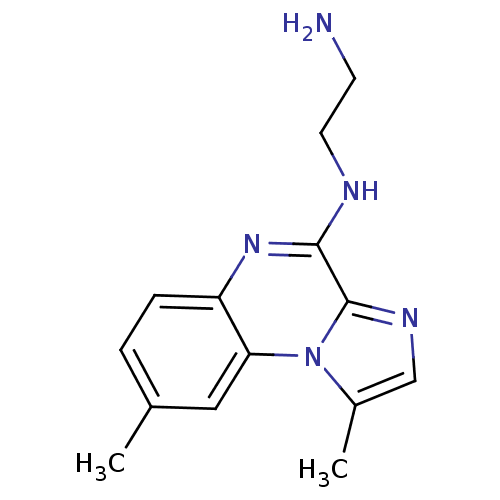
(BMS-345541 | BMS345541 | CHEMBL471496 | N-(2-amino...)Show InChI InChI=1S/C14H17N5/c1-9-3-4-11-12(7-9)19-10(2)8-17-14(19)13(18-11)16-6-5-15/h3-4,7-8H,5-6,15H2,1-2H3,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50355497
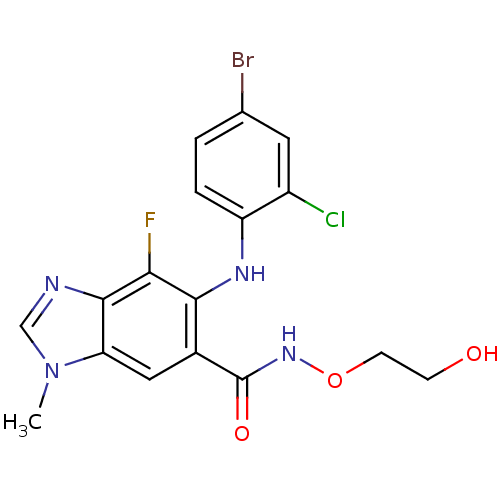
(AZD-6244 | CHEMBL1614701)Show SMILES Cn1cnc2c(F)c(Nc3ccc(Br)cc3Cl)c(cc12)C(=O)NOCCO Show InChI InChI=1S/C17H15BrClFN4O3/c1-24-8-21-16-13(24)7-10(17(26)23-27-5-4-25)15(14(16)20)22-12-3-2-9(18)6-11(12)19/h2-3,6-8,22,25H,4-5H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM21
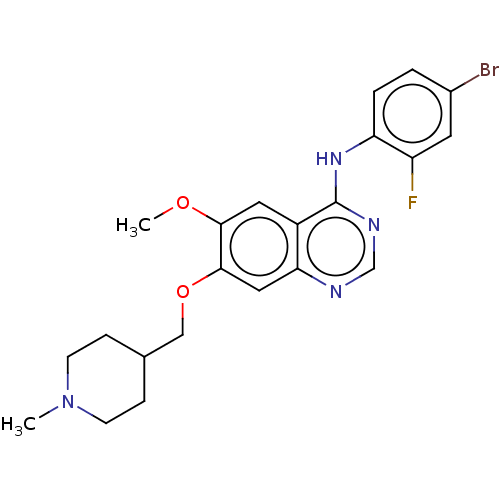
(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM25617
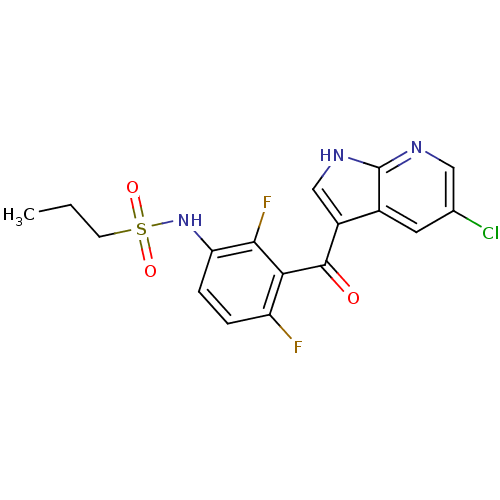
(N-[3-({5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl}carb...)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(C(=O)c2c[nH]c3ncc(Cl)cc23)c1F Show InChI InChI=1S/C17H14ClF2N3O3S/c1-2-5-27(25,26)23-13-4-3-12(19)14(15(13)20)16(24)11-8-22-17-10(11)6-9(18)7-21-17/h3-4,6-8,23H,2,5H2,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM482158
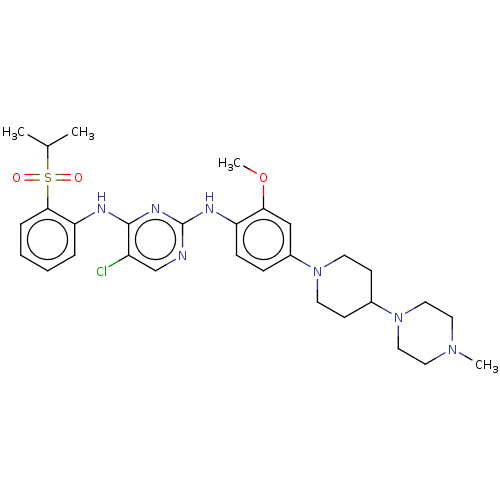
(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM24654
(4-N-(2,6-dichlorobenzene)-3-N-(piperidin-4-yl)-1H-...)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1cn[nH]c1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50355494
(CHEMBL1908396)Show SMILES COc1cc(Oc2ccnc3cc(OC)c(OC)cc23)ccc1NC(=O)NC(C)c1nccs1 Show InChI InChI=1S/C24H24N4O5S/c1-14(23-26-9-10-34-23)27-24(29)28-17-6-5-15(11-20(17)30-2)33-19-7-8-25-18-13-22(32-4)21(31-3)12-16(18)19/h5-14H,1-4H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM13530
(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50331096
(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-6-methoxy-7...)Show SMILES COc1cc2c(Oc3ccc4[nH]c(C)cc4c3F)ncnc2cc1OCCCN1CCCC1 Show InChI InChI=1S/C25H27FN4O3/c1-16-12-17-19(29-16)6-7-21(24(17)26)33-25-18-13-22(31-2)23(14-20(18)27-15-28-25)32-11-5-10-30-8-3-4-9-30/h6-7,12-15,29H,3-5,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
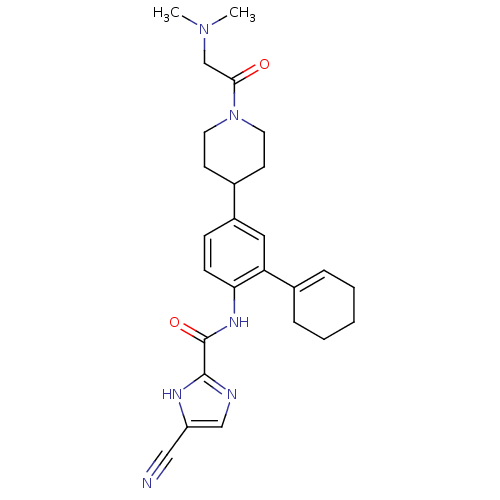
(Homo sapiens (Human)) | BDBM50355499
(CHEMBL1908395 | CHEMBL1908842)Show SMILES CN(C)CC(=O)N1CCC(CC1)c1ccc(NC(=O)c2ncc([nH]2)C#N)c(c1)C1=CCCCC1 |t:31| Show InChI InChI=1S/C26H32N6O2/c1-31(2)17-24(33)32-12-10-18(11-13-32)20-8-9-23(22(14-20)19-6-4-3-5-7-19)30-26(34)25-28-16-21(15-27)29-25/h6,8-9,14,16,18H,3-5,7,10-13,17H2,1-2H3,(H,28,29)(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
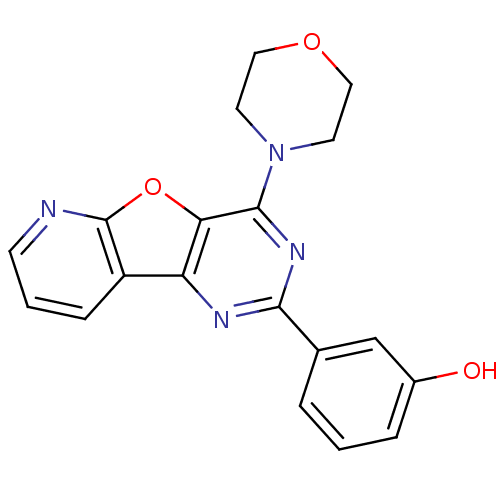
(Homo sapiens (Human)) | BDBM25045
(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
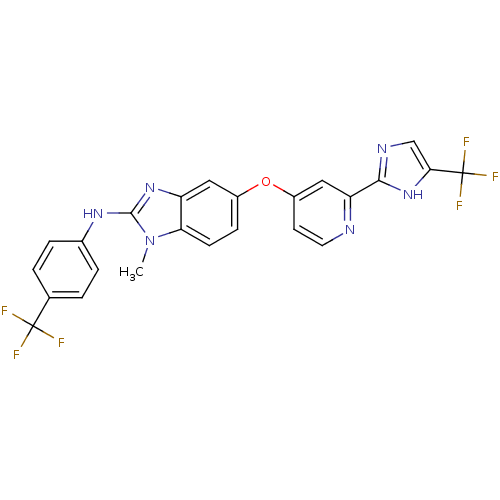
(Homo sapiens (Human)) | BDBM31088
(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
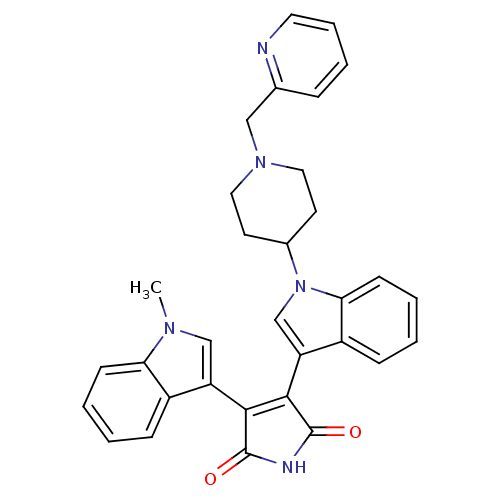
(Homo sapiens (Human)) | BDBM50128285
(3-(1-Methyl-1H-indol-3-yl)-4-[1-(1-pyridin-2-ylmet...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(C3CCN(Cc4ccccn4)CC3)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C32H29N5O2/c1-35-19-25(23-9-2-4-11-27(23)35)29-30(32(39)34-31(29)38)26-20-37(28-12-5-3-10-24(26)28)22-13-16-36(17-14-22)18-21-8-6-7-15-33-21/h2-12,15,19-20,22H,13-14,16-18H2,1H3,(H,34,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for BTK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data