 Found 271 hits of ki data for polymerid = 1846,50006599
Found 271 hits of ki data for polymerid = 1846,50006599 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206362
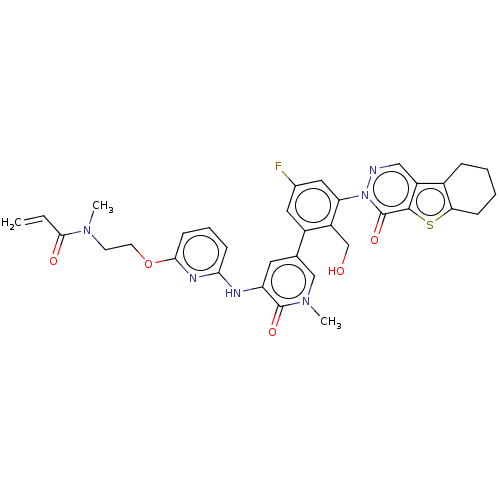
(US9260415, 113)Show SMILES CN(CCOc1cccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(c2CO)-n2ncc3c4CCCCc4sc3c2=O)n1)C(=O)C=C Show InChI InChI=1S/C34H33FN6O5S/c1-4-31(43)39(2)12-13-46-30-11-7-10-29(38-30)37-26-14-20(18-40(3)33(26)44)23-15-21(35)16-27(25(23)19-42)41-34(45)32-24(17-36-41)22-8-5-6-9-28(22)47-32/h4,7,10-11,14-18,42H,1,5-6,8-9,12-13,19H2,2-3H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206354
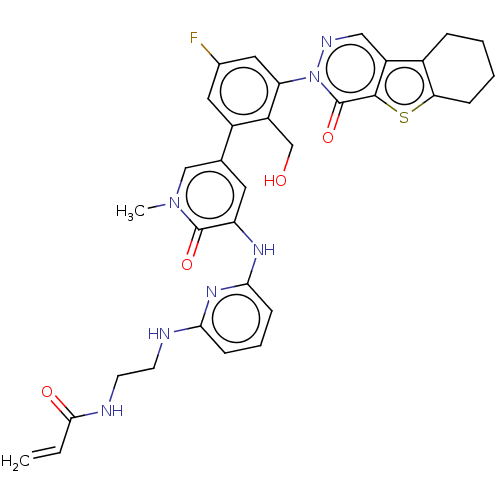
(US9260415, 105)Show SMILES Cn1cc(cc(Nc2cccc(NCCNC(=O)C=C)n2)c1=O)-c1cc(F)cc(c1CO)-n1ncc2c3CCCCc3sc2c1=O Show InChI InChI=1S/C33H32FN7O4S/c1-3-30(43)36-12-11-35-28-9-6-10-29(39-28)38-25-13-19(17-40(2)32(25)44)22-14-20(34)15-26(24(22)18-42)41-33(45)31-23(16-37-41)21-7-4-5-8-27(21)46-31/h3,6,9-10,13-17,42H,1,4-5,7-8,11-12,18H2,2H3,(H,36,43)(H2,35,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.248 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50245852
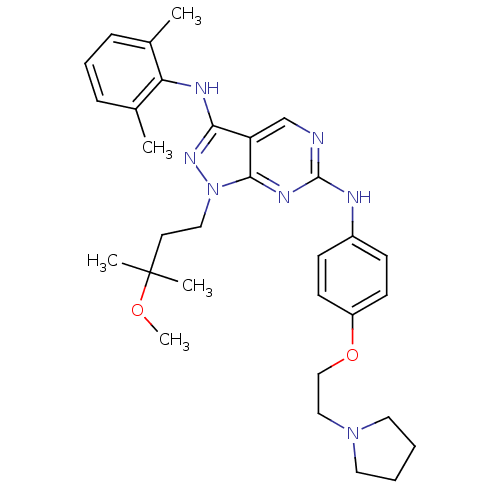
(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206355

(US9260415, 106)Show SMILES CC#CC(=O)NCCOc1cccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(c2CO)-n2ncc3c4CCCCc4sc3c2=O)n1 Show InChI InChI=1S/C34H31FN6O5S/c1-3-7-30(43)36-12-13-46-31-11-6-10-29(39-31)38-26-14-20(18-40(2)33(26)44)23-15-21(35)16-27(25(23)19-42)41-34(45)32-24(17-37-41)22-8-4-5-9-28(22)47-32/h6,10-11,14-18,42H,4-5,8-9,12-13,19H2,1-2H3,(H,36,43)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.306 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244490
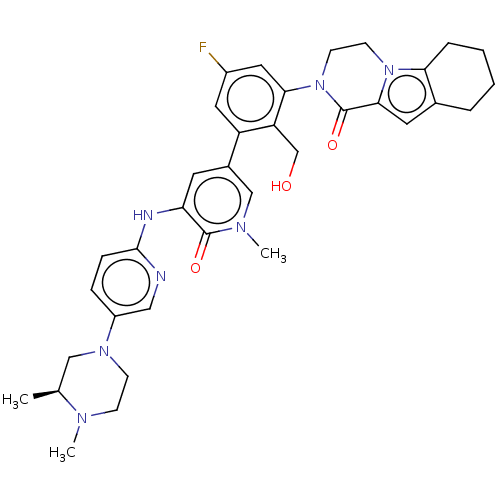
(CHEMBL4102992)Show SMILES C[C@H]1CN(CCN1C)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-41(11-10-39(22)2)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111951
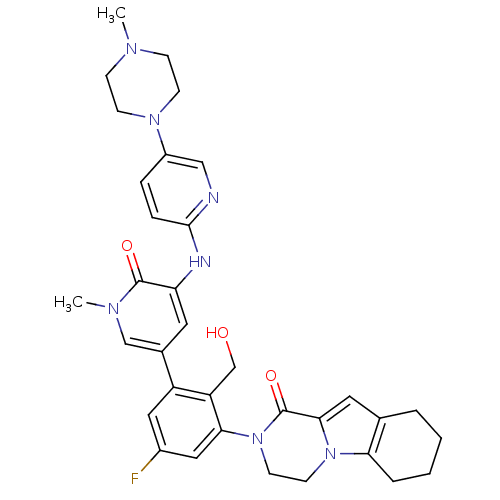
(US8618107, 197)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C34H38FN7O3/c1-38-9-11-40(12-10-38)25-7-8-32(36-19-25)37-28-15-23(20-39(2)33(28)44)26-17-24(35)18-30(27(26)21-43)42-14-13-41-29-6-4-3-5-22(29)16-31(41)34(42)45/h7-8,15-20,43H,3-6,9-14,21H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244489

(CHEMBL4095379)Show SMILES C[C@@H]1CN(C)CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-39(2)10-11-41(22)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244488
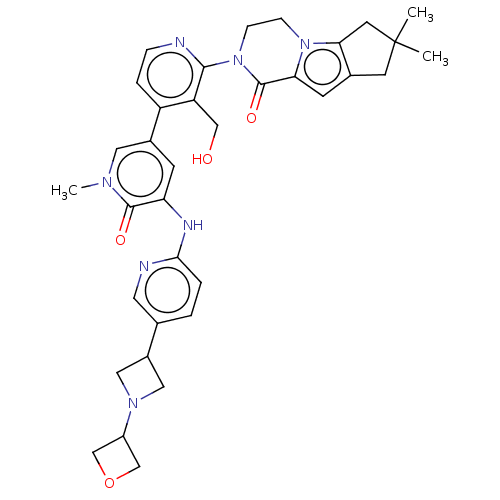
(CHEMBL4069790)Show SMILES Cn1cc(cc(Nc2ccc(cn2)C2CN(C2)C2COC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C35H39N7O4/c1-35(2)12-22-11-29-34(45)42(9-8-41(29)30(22)13-35)32-27(18-43)26(6-7-36-32)23-10-28(33(44)39(3)15-23)38-31-5-4-21(14-37-31)24-16-40(17-24)25-19-46-20-25/h4-7,10-11,14-15,24-25,43H,8-9,12-13,16-20H2,1-3H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244491
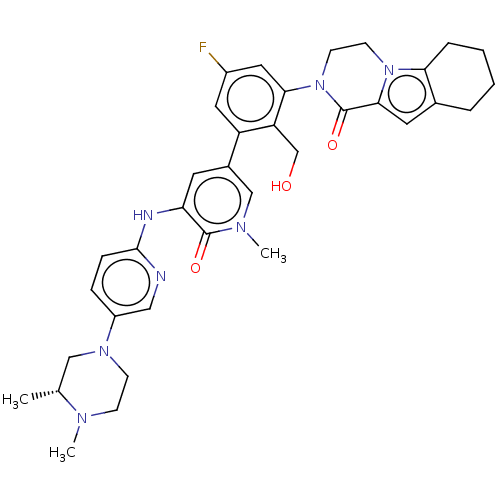
(CHEMBL4092794)Show SMILES C[C@@H]1CN(CCN1C)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-41(11-10-39(22)2)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244494
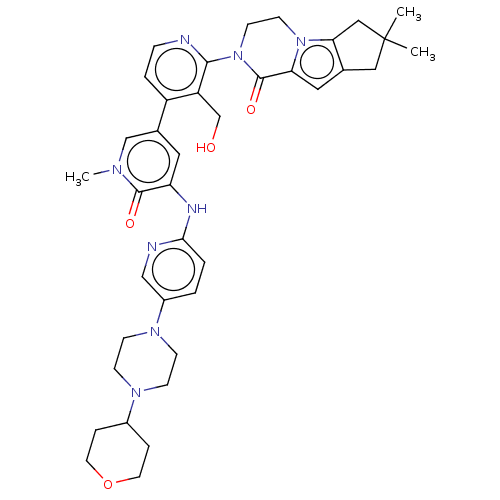
(CHEMBL4090117)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2CCOCC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C38H46N8O4/c1-38(2)20-25-19-32-37(49)46(15-14-45(32)33(25)21-38)35-30(24-47)29(6-9-39-35)26-18-31(36(48)42(3)23-26)41-34-5-4-28(22-40-34)44-12-10-43(11-13-44)27-7-16-50-17-8-27/h4-6,9,18-19,22-23,27,47H,7-8,10-17,20-21,24H2,1-3H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206360

(US9260415, 111)Show SMILES CN(CCNC(=O)C=C)c1cccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(c2CO)-n2ncc3c4CCCCc4sc3c2=O)n1 Show InChI InChI=1S/C34H34FN7O4S/c1-4-31(44)36-12-13-40(2)30-11-7-10-29(39-30)38-26-14-20(18-41(3)33(26)45)23-15-21(35)16-27(25(23)19-43)42-34(46)32-24(17-37-42)22-8-5-6-9-28(22)47-32/h4,7,10-11,14-18,43H,1,5-6,8-9,12-13,19H2,2-3H3,(H,36,44)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.494 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206366
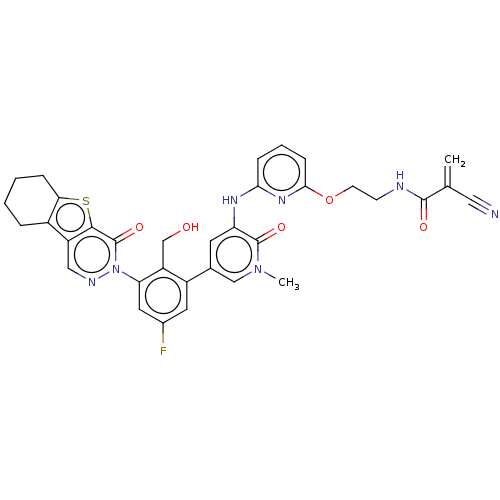
(US9260415, 117)Show SMILES Cn1cc(cc(Nc2cccc(OCCNC(=O)C(=C)C#N)n2)c1=O)-c1cc(F)cc(c1CO)-n1ncc2c3CCCCc3sc2c1=O Show InChI InChI=1S/C34H30FN7O5S/c1-19(15-36)32(44)37-10-11-47-30-9-5-8-29(40-30)39-26-12-20(17-41(2)33(26)45)23-13-21(35)14-27(25(23)18-43)42-34(46)31-24(16-38-42)22-6-3-4-7-28(22)48-31/h5,8-9,12-14,16-17,43H,1,3-4,6-7,10-11,18H2,2H3,(H,37,44)(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.576 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244467
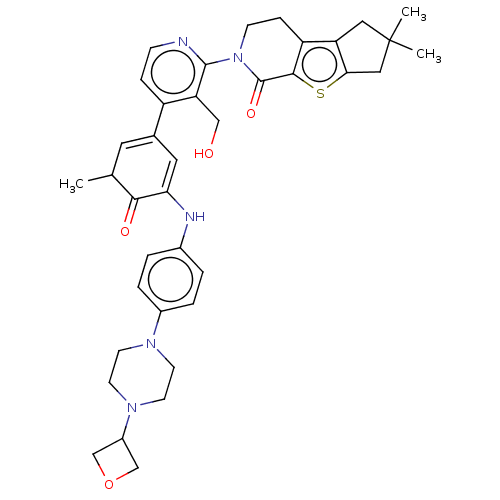
(CHEMBL4063638)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO |c:2,t:4| Show InChI InChI=1S/C38H43N5O4S/c1-23-16-24(17-32(34(23)45)40-25-4-6-26(7-5-25)41-12-14-42(15-13-41)27-21-47-22-27)28-8-10-39-36(31(28)20-44)43-11-9-29-30-18-38(2,3)19-33(30)48-35(29)37(43)46/h4-8,10,16-17,23,27,40,44H,9,11-15,18-22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206356
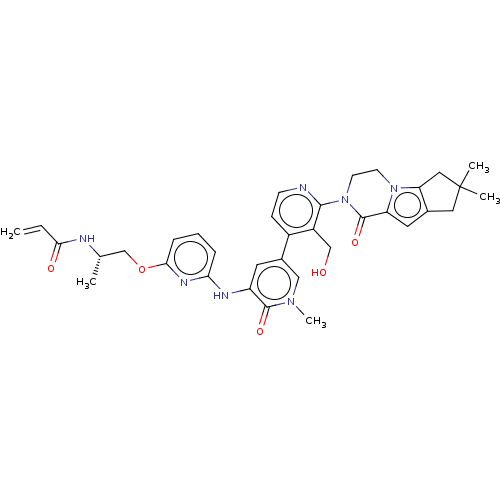
(US9260415, 107)Show SMILES C[C@@H](COc1cccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)n1)NC(=O)C=C |r| Show InChI InChI=1S/C35H39N7O5/c1-6-30(44)37-21(2)20-47-31-9-7-8-29(39-31)38-26-14-23(18-40(5)33(26)45)24-10-11-36-32(25(24)19-43)42-13-12-41-27(34(42)46)15-22-16-35(3,4)17-28(22)41/h6-11,14-15,18,21,43H,1,12-13,16-17,19-20H2,2-5H3,(H,37,44)(H,38,39)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.612 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244502

(CHEMBL4085043)Show SMILES C[C@H]1CN(C)CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-39(2)10-11-41(22)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206359
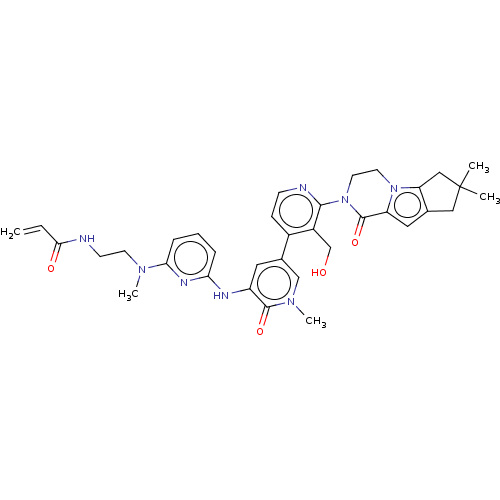
(US9260415, 110)Show SMILES CN(CCNC(=O)C=C)c1cccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)n1 Show InChI InChI=1S/C35H40N8O4/c1-6-31(45)36-12-13-40(4)30-9-7-8-29(39-30)38-26-16-23(20-41(5)33(26)46)24-10-11-37-32(25(24)21-44)43-15-14-42-27(34(43)47)17-22-18-35(2,3)19-28(22)42/h6-11,16-17,20,44H,1,12-15,18-19,21H2,2-5H3,(H,36,45)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.683 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111952
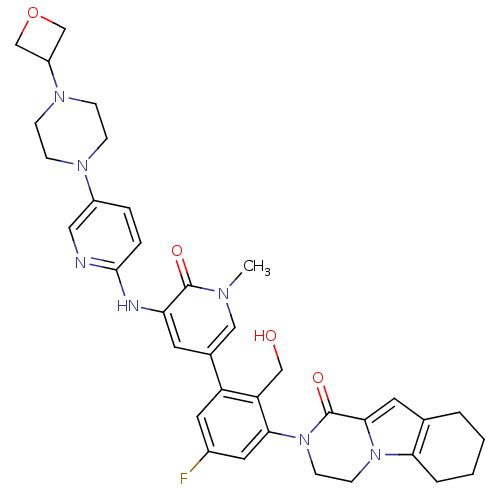
(US8618107, 210)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1cc(F)cc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C36H40FN7O4/c1-40-19-24(14-30(35(40)46)39-34-7-6-26(18-38-34)41-8-10-42(11-9-41)27-21-48-22-27)28-16-25(37)17-32(29(28)20-45)44-13-12-43-31-5-3-2-4-23(31)15-33(43)36(44)47/h6-7,14-19,27,45H,2-5,8-13,20-22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206350
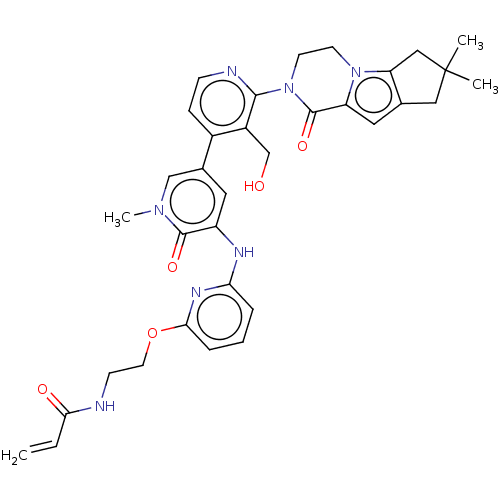
(US9260415, 101)Show SMILES Cn1cc(cc(Nc2cccc(OCCNC(=O)C=C)n2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C34H37N7O5/c1-5-29(43)35-11-14-46-30-8-6-7-28(38-30)37-25-15-22(19-39(4)32(25)44)23-9-10-36-31(24(23)20-42)41-13-12-40-26(33(41)45)16-21-17-34(2,3)18-27(21)40/h5-10,15-16,19,42H,1,11-14,17-18,20H2,2-4H3,(H,35,43)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.792 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244493
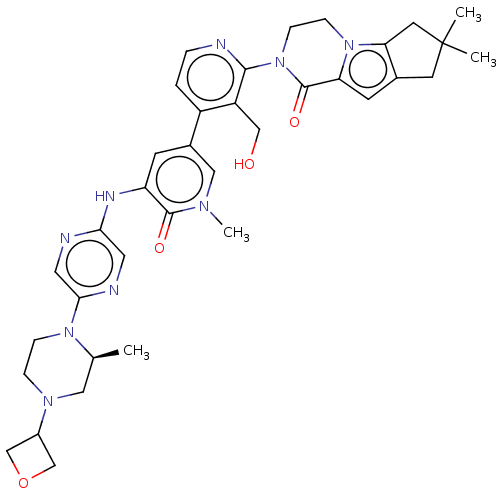
(CHEMBL4070991)Show SMILES C[C@H]1CN(CCN1c1cnc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)cn1)C1COC1 |r| Show InChI InChI=1S/C36H43N9O4/c1-22-17-42(25-20-49-21-25)7-8-43(22)32-16-38-31(15-39-32)40-28-11-24(18-41(4)34(28)47)26-5-6-37-33(27(26)19-46)45-10-9-44-29(35(45)48)12-23-13-36(2,3)14-30(23)44/h5-6,11-12,15-16,18,22,25,46H,7-10,13-14,17,19-21H2,1-4H3,(H,38,40)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206351
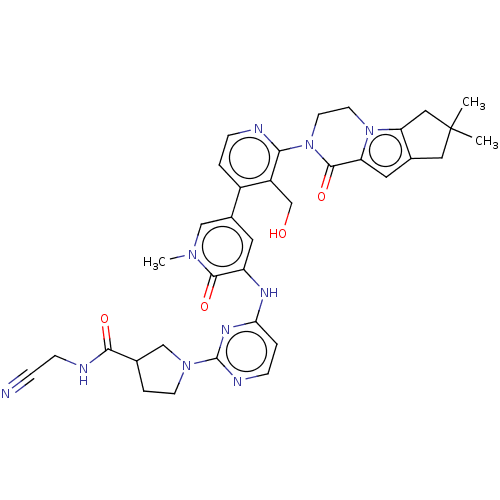
(US9260415, 102)Show SMILES Cn1cc(cc(Nc2ccnc(n2)N2CCC(C2)C(=O)NCC#N)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C35H38N10O4/c1-35(2)16-22-15-27-33(49)45(13-12-44(27)28(22)17-35)30-25(20-46)24(4-8-37-30)23-14-26(32(48)42(3)18-23)40-29-5-9-39-34(41-29)43-11-6-21(19-43)31(47)38-10-7-36/h4-5,8-9,14-15,18,21,46H,6,10-13,16-17,19-20H2,1-3H3,(H,38,47)(H,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.864 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206363

(US9260415, 114)Show SMILES Cn1cc(cc(Nc2cccc(n2)N2CCC[C@@H](C2)NC(=O)C=C)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO |r| Show InChI InChI=1S/C37H42N8O4/c1-5-33(47)39-25-8-7-13-43(21-25)32-10-6-9-31(41-32)40-28-16-24(20-42(4)35(28)48)26-11-12-38-34(27(26)22-46)45-15-14-44-29(36(45)49)17-23-18-37(2,3)19-30(23)44/h5-6,9-12,16-17,20,25,46H,1,7-8,13-15,18-19,21-22H2,2-4H3,(H,39,47)(H,40,41)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.888 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244440
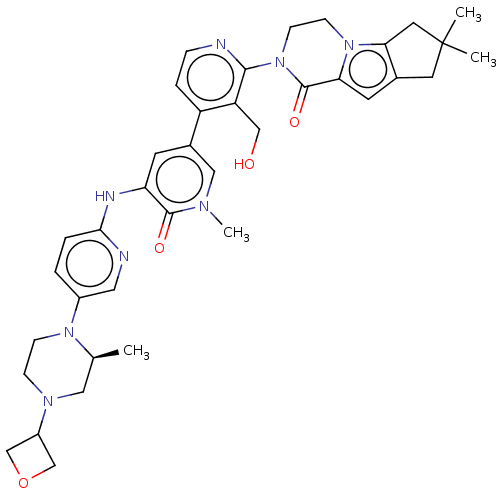
(CHEMBL4065122)Show SMILES C[C@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114009
BindingDB Entry DOI: 10.7270/Q2ZK5MN1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244440

(CHEMBL4065122)Show SMILES C[C@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206353
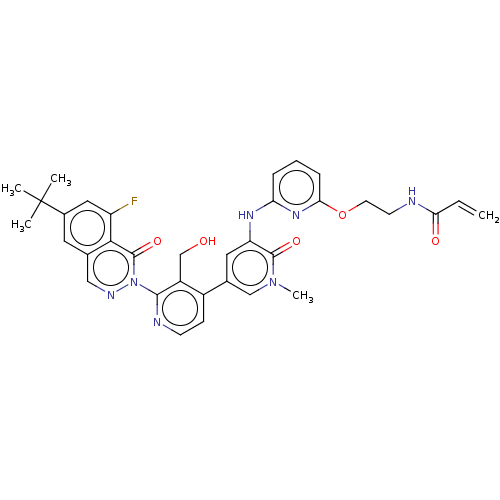
(US9260415, 104)Show SMILES Cn1cc(cc(Nc2cccc(OCCNC(=O)C=C)n2)c1=O)-c1ccnc(c1CO)-n1ncc2cc(cc(F)c2c1=O)C(C)(C)C Show InChI InChI=1S/C34H34FN7O5/c1-6-28(44)36-12-13-47-29-9-7-8-27(40-29)39-26-15-21(18-41(5)32(26)45)23-10-11-37-31(24(23)19-43)42-33(46)30-20(17-38-42)14-22(16-25(30)35)34(2,3)4/h6-11,14-18,43H,1,12-13,19H2,2-5H3,(H,36,44)(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.915 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244492
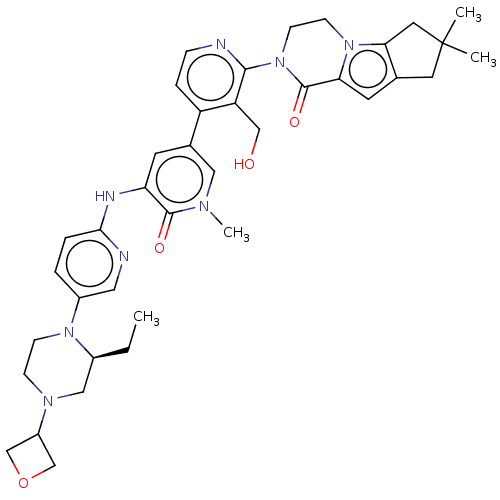
(CHEMBL4087543)Show SMILES CC[C@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C38H46N8O4/c1-5-26-20-43(28-22-50-23-28)10-11-44(26)27-6-7-34(40-18-27)41-31-14-25(19-42(4)36(31)48)29-8-9-39-35(30(29)21-47)46-13-12-45-32(37(46)49)15-24-16-38(2,3)17-33(24)45/h6-9,14-15,18-19,26,28,47H,5,10-13,16-17,20-23H2,1-4H3,(H,40,41)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244486

(CHEMBL4097832)Show SMILES COCCCN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C37H46N8O4/c1-37(2)20-25-19-31-36(48)45(16-15-44(31)32(25)21-37)34-29(24-46)28(8-9-38-34)26-18-30(35(47)41(3)23-26)40-33-7-6-27(22-39-33)43-13-11-42(12-14-43)10-5-17-49-4/h6-9,18-19,22-23,46H,5,10-17,20-21,24H2,1-4H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244500
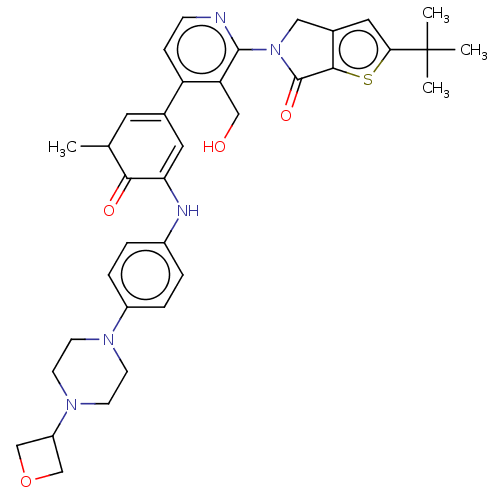
(CHEMBL4093188)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2Cc3cc(sc3C2=O)C(C)(C)C)c1CO |c:2,t:4| Show InChI InChI=1S/C36H41N5O4S/c1-22-15-23(28-9-10-37-34(29(28)19-42)41-18-24-17-31(36(2,3)4)46-33(24)35(41)44)16-30(32(22)43)38-25-5-7-26(8-6-25)39-11-13-40(14-12-39)27-20-45-21-27/h5-10,15-17,22,27,38,42H,11-14,18-21H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244501
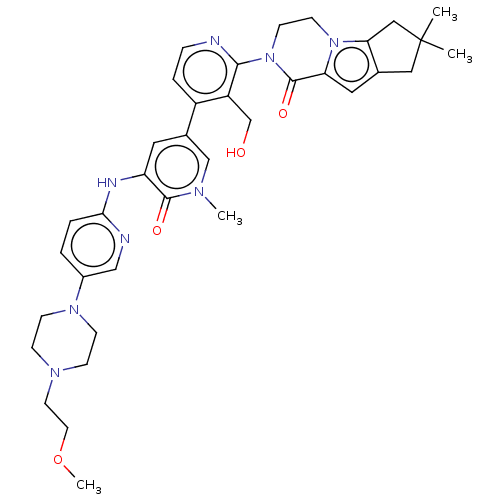
(CHEMBL4062634)Show SMILES COCCN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C36H44N8O4/c1-36(2)19-24-18-30-35(47)44(14-13-43(30)31(24)20-36)33-28(23-45)27(7-8-37-33)25-17-29(34(46)40(3)22-25)39-32-6-5-26(21-38-32)42-11-9-41(10-12-42)15-16-48-4/h5-8,17-18,21-22,45H,9-16,19-20,23H2,1-4H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244497

(CHEMBL4074792)Show SMILES C[C@@H]1CN([C@@H](C)CN1C1COC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C38H46N8O4/c1-23-18-46(28-21-50-22-28)24(2)17-45(23)27-6-7-34(40-16-27)41-31-12-26(19-42(5)36(31)48)29-8-9-39-35(30(29)20-47)44-11-10-43-32(37(44)49)13-25-14-38(3,4)15-33(25)43/h6-9,12-13,16,19,23-24,28,47H,10-11,14-15,17-18,20-22H2,1-5H3,(H,40,41)/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111939

(US8618107, 105)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO Show InChI InChI=1S/C29H29N5O3S/c1-29(2)12-20-19-8-10-34(28(37)26(19)38-24(20)13-29)23-6-4-5-18(21(23)15-35)17-11-22(27(36)33(3)14-17)32-25-7-9-30-16-31-25/h4-7,9,11,14,16,35H,8,10,12-13,15H2,1-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244440

(CHEMBL4065122)Show SMILES C[C@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114009
BindingDB Entry DOI: 10.7270/Q2ZK5MN1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206367
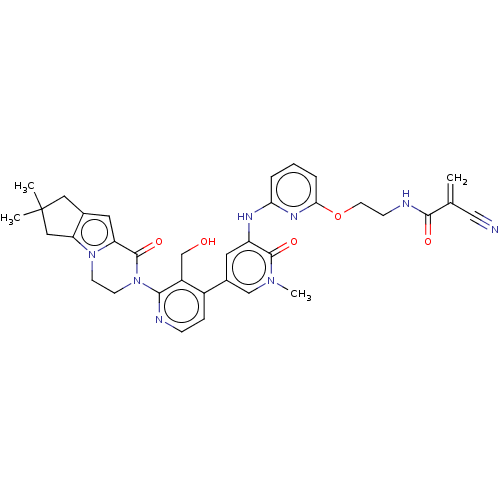
(US9260415, 118)Show SMILES Cn1cc(cc(Nc2cccc(OCCNC(=O)C(=C)C#N)n2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C35H36N8O5/c1-21(18-36)32(45)38-10-13-48-30-7-5-6-29(40-30)39-26-14-23(19-41(4)33(26)46)24-8-9-37-31(25(24)20-44)43-12-11-42-27(34(43)47)15-22-16-35(2,3)17-28(22)42/h5-9,14-15,19,44H,1,10-13,16-17,20H2,2-4H3,(H,38,45)(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206361

(US9260415, 112)Show SMILES CN(CCOc1cccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)n1)C(=O)C=C Show InChI InChI=1S/C35H39N7O5/c1-6-31(44)39(4)14-15-47-30-9-7-8-29(38-30)37-26-16-23(20-40(5)33(26)45)24-10-11-36-32(25(24)21-43)42-13-12-41-27(34(42)46)17-22-18-35(2,3)19-28(22)41/h6-11,16-17,20,43H,1,12-15,18-19,21H2,2-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244495
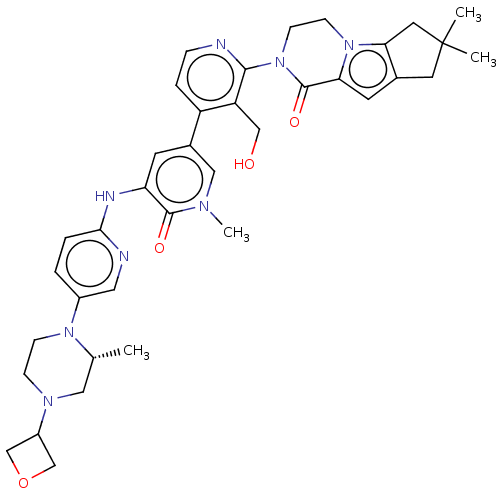
(CHEMBL4066176)Show SMILES C[C@@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244484
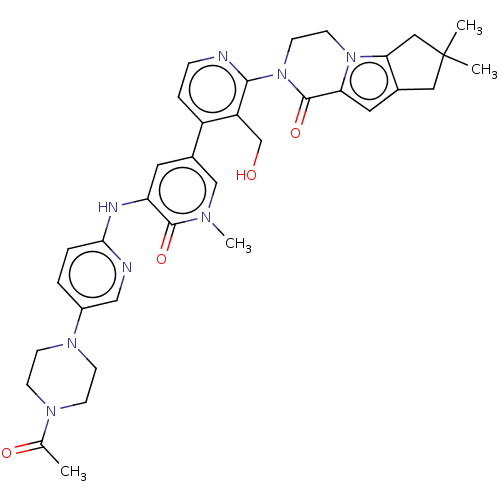
(CHEMBL4060356)Show SMILES CC(=O)N1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C35H40N8O4/c1-22(45)40-9-11-41(12-10-40)25-5-6-31(37-19-25)38-28-15-24(20-39(4)33(28)46)26-7-8-36-32(27(26)21-44)43-14-13-42-29(34(43)47)16-23-17-35(2,3)18-30(23)42/h5-8,15-16,19-20,44H,9-14,17-18,21H2,1-4H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244483
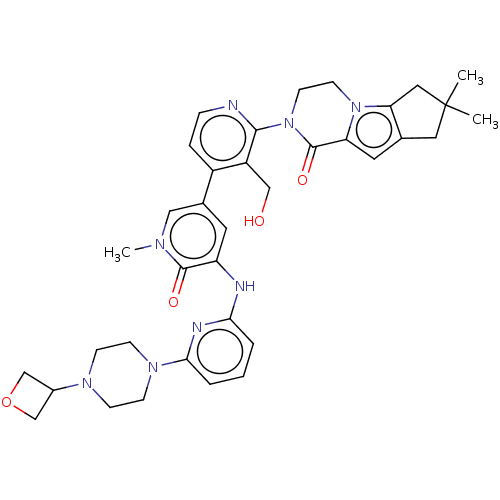
(CHEMBL4082268)Show SMILES Cn1cc(cc(Nc2cccc(n2)N2CCN(CC2)C2COC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C36H42N8O4/c1-36(2)17-23-16-29-35(47)44(14-13-43(29)30(23)18-36)33-27(20-45)26(7-8-37-33)24-15-28(34(46)40(3)19-24)38-31-5-4-6-32(39-31)42-11-9-41(10-12-42)25-21-48-22-25/h4-8,15-16,19,25,45H,9-14,17-18,20-22H2,1-3H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
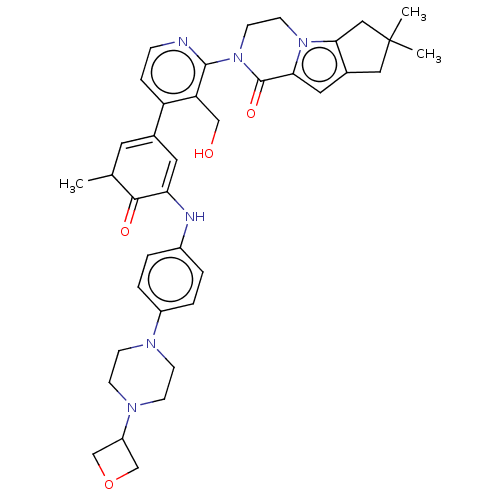
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244499
(CHEMBL4086408)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO |c:2,t:4| Show InChI InChI=1S/C38H44N6O4/c1-24-16-25(17-32(35(24)46)40-27-4-6-28(7-5-27)41-10-12-42(13-11-41)29-22-48-23-29)30-8-9-39-36(31(30)21-45)44-15-14-43-33(37(44)47)18-26-19-38(2,3)20-34(26)43/h4-9,16-18,24,29,40,45H,10-15,19-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
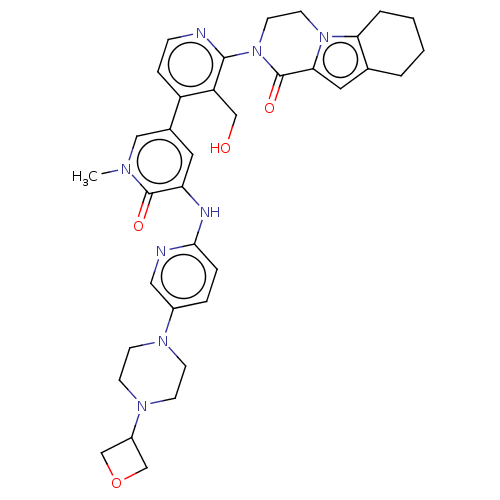
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244464
(CHEMBL4085477)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1ccnc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C35H40N8O4/c1-39-19-24(16-29(34(39)45)38-32-7-6-25(18-37-32)40-10-12-41(13-11-40)26-21-47-22-26)27-8-9-36-33(28(27)20-44)43-15-14-42-30-5-3-2-4-23(30)17-31(42)35(43)46/h6-9,16-19,26,44H,2-5,10-15,20-22H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
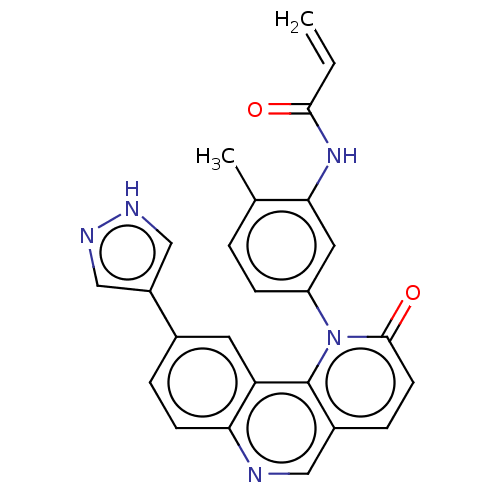
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM400813
(QL-X-138 | US10000483, Compound II-6)Show SMILES Cc1ccc(cc1NC(=O)C=C)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1cn[nH]c1 Show InChI InChI=1S/C25H19N5O2/c1-3-23(31)29-22-11-19(7-4-15(22)2)30-24(32)9-6-17-12-26-21-8-5-16(10-20(21)25(17)30)18-13-27-28-14-18/h3-14H,1H2,2H3,(H,27,28)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China
Curated by ChEMBL
| Assay Description
Irreversible inhibition of recombinant full length His-tagged human BTK expressed in baculovirus expression system using poly (4:1 Glu, Tyr) as subst... |
Eur J Med Chem 137: 545-557 (2017)
Article DOI: 10.1016/j.ejmech.2017.06.016
BindingDB Entry DOI: 10.7270/Q2NG4T51 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206364
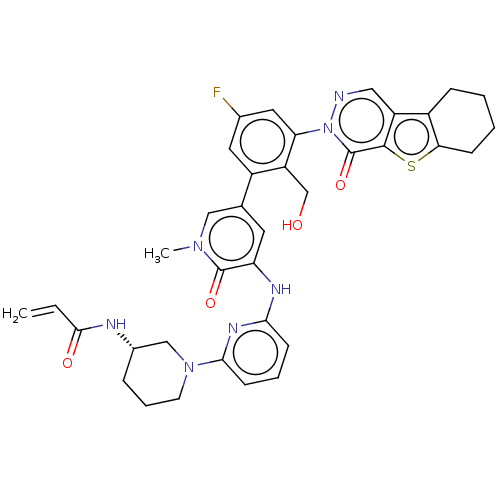
(US9260415, 115)Show SMILES Cn1cc(cc(Nc2cccc(n2)N2CCC[C@@H](C2)NC(=O)C=C)c1=O)-c1cc(F)cc(c1CO)-n1ncc2c3CCCCc3sc2c1=O |r| Show InChI InChI=1S/C36H36FN7O4S/c1-3-33(46)39-23-8-7-13-43(19-23)32-12-6-11-31(41-32)40-28-14-21(18-42(2)35(28)47)25-15-22(37)16-29(27(25)20-45)44-36(48)34-26(17-38-44)24-9-4-5-10-30(24)49-34/h3,6,11-12,14-18,23,45H,1,4-5,7-10,13,19-20H2,2H3,(H,39,46)(H,40,41)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.16 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
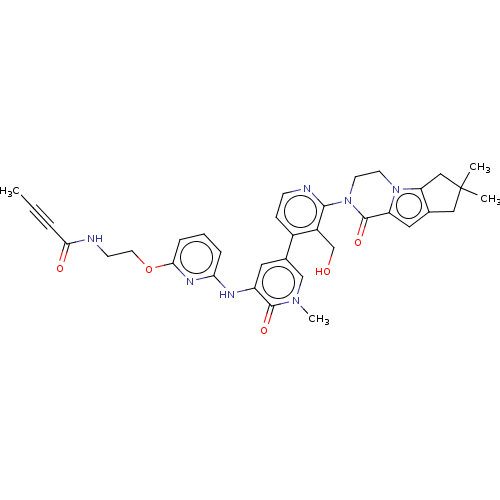
(Homo sapiens (Human)) | BDBM206365
(US9260415, 116)Show SMILES CC#CC(=O)NCCOc1cccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)n1 Show InChI InChI=1S/C35H37N7O5/c1-5-7-30(44)36-12-15-47-31-9-6-8-29(39-31)38-26-16-23(20-40(4)33(26)45)24-10-11-37-32(25(24)21-43)42-14-13-41-27(34(42)46)17-22-18-35(2,3)19-28(22)41/h6,8-11,16-17,20,43H,12-15,18-19,21H2,1-4H3,(H,36,44)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.37 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
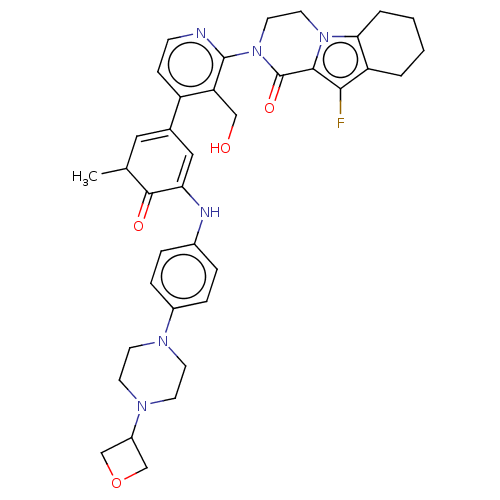
(Homo sapiens (Human)) | BDBM50244469
(CHEMBL4090946)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2CCn3c4CCCCc4c(F)c3C2=O)c1CO |c:2,t:4| Show InChI InChI=1S/C37H41FN6O4/c1-23-18-24(19-31(35(23)46)40-25-6-8-26(9-7-25)41-12-14-42(15-13-41)27-21-48-22-27)28-10-11-39-36(30(28)20-45)44-17-16-43-32-5-3-2-4-29(32)33(38)34(43)37(44)47/h6-11,18-19,23,27,40,45H,2-5,12-17,20-22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
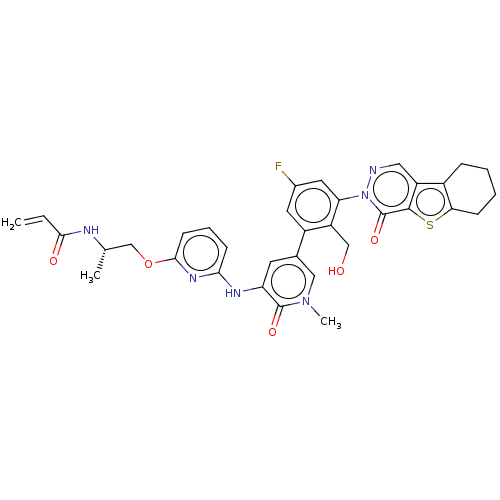
(Homo sapiens (Human)) | BDBM206357
(US9260415, 108)Show SMILES C[C@@H](COc1cccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(c2CO)-n2ncc3c4CCCCc4sc3c2=O)n1)NC(=O)C=C |r| Show InChI InChI=1S/C34H33FN6O5S/c1-4-30(43)37-19(2)18-46-31-11-7-10-29(39-31)38-26-12-20(16-40(3)33(26)44)23-13-21(35)14-27(25(23)17-42)41-34(45)32-24(15-36-41)22-8-5-6-9-28(22)47-32/h4,7,10-16,19,42H,1,5-6,8-9,17-18H2,2-3H3,(H,37,43)(H,38,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.57 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM102620
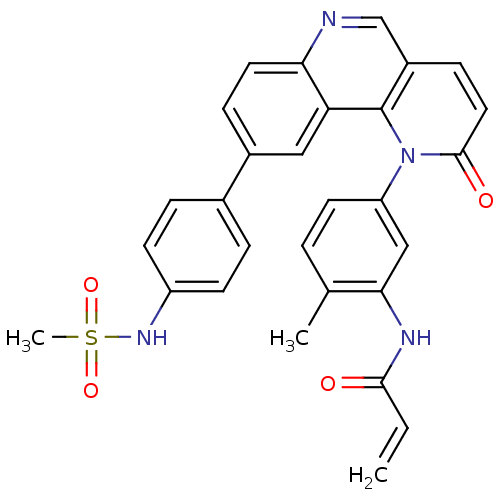
(BMX-IN-1 | N-[5-[9-[4-(methanesulfonamido)phenyl]-...)Show SMILES Cc1ccc(cc1NC(=O)C=C)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C29H24N4O4S/c1-4-27(34)31-26-16-23(12-5-18(26)2)33-28(35)14-9-21-17-30-25-13-8-20(15-24(25)29(21)33)19-6-10-22(11-7-19)32-38(3,36)37/h4-17,32H,1H2,2-3H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China
Curated by ChEMBL
| Assay Description
Irreversible inhibition of recombinant full length His-tagged human BTK expressed in baculovirus expression system using poly (4:1 Glu, Tyr) as subst... |
Eur J Med Chem 137: 545-557 (2017)
Article DOI: 10.1016/j.ejmech.2017.06.016
BindingDB Entry DOI: 10.7270/Q2NG4T51 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244485

(CHEMBL4071754)Show SMILES CC(=O)N1CC2(C1)CN(C2)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C36H40N8O4/c1-22(46)41-18-36(19-41)20-42(21-36)25-5-6-31(38-15-25)39-28-11-24(16-40(4)33(28)47)26-7-8-37-32(27(26)17-45)44-10-9-43-29(34(44)48)12-23-13-35(2,3)14-30(23)43/h5-8,11-12,15-16,45H,9-10,13-14,17-21H2,1-4H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244487
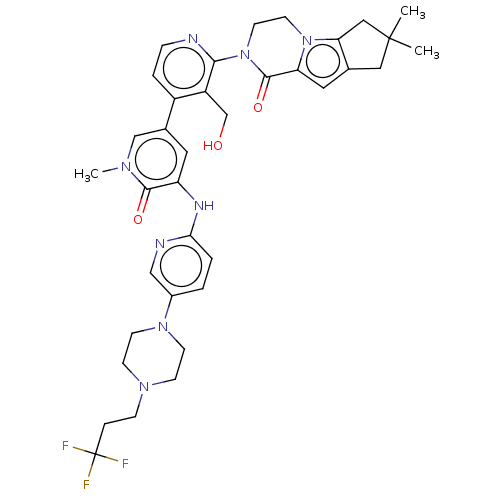
(CHEMBL4079803)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CCC(F)(F)F)CC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C36H41F3N8O3/c1-35(2)18-23-17-29-34(50)47(15-14-46(29)30(23)19-35)32-27(22-48)26(6-8-40-32)24-16-28(33(49)43(3)21-24)42-31-5-4-25(20-41-31)45-12-10-44(11-13-45)9-7-36(37,38)39/h4-6,8,16-17,20-21,48H,7,9-15,18-19,22H2,1-3H3,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244468
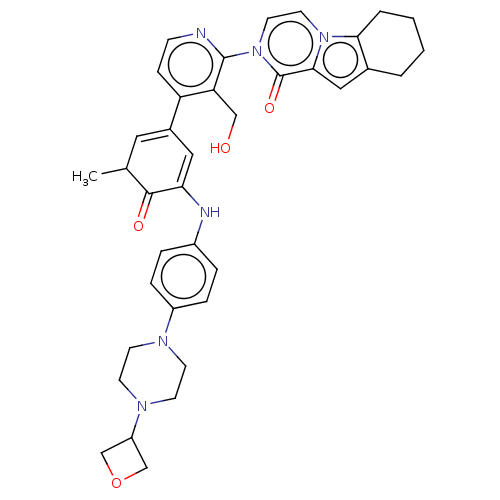
(CHEMBL4104307)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(c1CO)-n1ccn2c3CCCCc3cc2c1=O |c:2,t:4| Show InChI InChI=1S/C37H40N6O4/c1-24-18-26(19-32(35(24)45)39-27-6-8-28(9-7-27)40-12-14-41(15-13-40)29-22-47-23-29)30-10-11-38-36(31(30)21-44)43-17-16-42-33-5-3-2-4-25(33)20-34(42)37(43)46/h6-11,16-20,24,29,39,44H,2-5,12-15,21-23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM206358
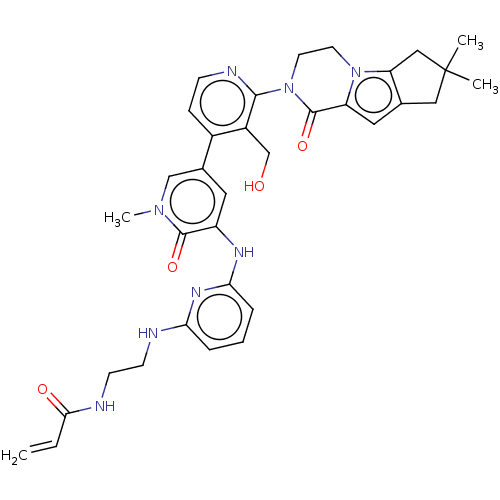
(US9260415, 109)Show SMILES Cn1cc(cc(Nc2cccc(NCCNC(=O)C=C)n2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C34H38N8O4/c1-5-30(44)36-12-11-35-28-7-6-8-29(39-28)38-25-15-22(19-40(4)32(25)45)23-9-10-37-31(24(23)20-43)42-14-13-41-26(33(42)46)16-21-17-34(2,3)18-27(21)41/h5-10,15-16,19,43H,1,11-14,17-18,20H2,2-4H3,(H,36,44)(H2,35,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.33 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
US Patent
| Assay Description
A generalized procedure for a standard biochemical Btk, Kinase Assay that can be used to test Formula I compounds is as follows. A master mix minus B... |
US Patent US9260415 (2016)
BindingDB Entry DOI: 10.7270/Q2WH2NT7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China
Curated by ChEMBL
| Assay Description
Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... |
Eur J Med Chem 131: 107-125 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.001
BindingDB Entry DOI: 10.7270/Q21V5HCK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244466
(CHEMBL4075845)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2CCc3c4CCCCc4sc3C2=O)c1CO |c:2,t:4| Show InChI InChI=1S/C37H41N5O4S/c1-23-18-24(19-32(34(23)44)39-25-6-8-26(9-7-25)40-14-16-41(17-15-40)27-21-46-22-27)28-10-12-38-36(31(28)20-43)42-13-11-30-29-4-2-3-5-33(29)47-35(30)37(42)45/h6-10,12,18-19,23,27,39,43H,2-5,11,13-17,20-22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data