 Found 8 hits of ki for UniProtKB: P04626
Found 8 hits of ki for UniProtKB: P04626 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50236369
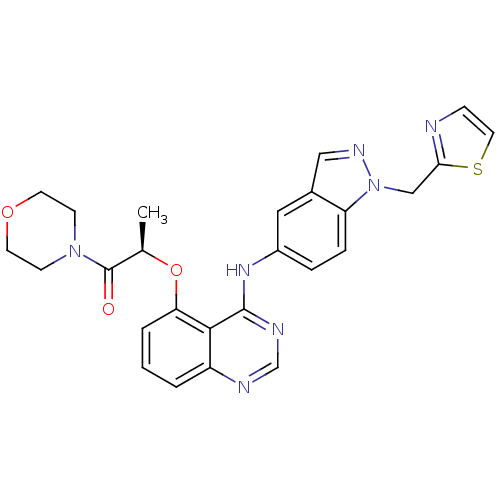
((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5nccs5)ncc4c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H25N7O3S/c1-17(26(34)32-8-10-35-11-9-32)36-22-4-2-3-20-24(22)25(29-16-28-20)31-19-5-6-21-18(13-19)14-30-33(21)15-23-27-7-12-37-23/h2-7,12-14,16-17H,8-11,15H2,1H3,(H,28,29,31)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50555575
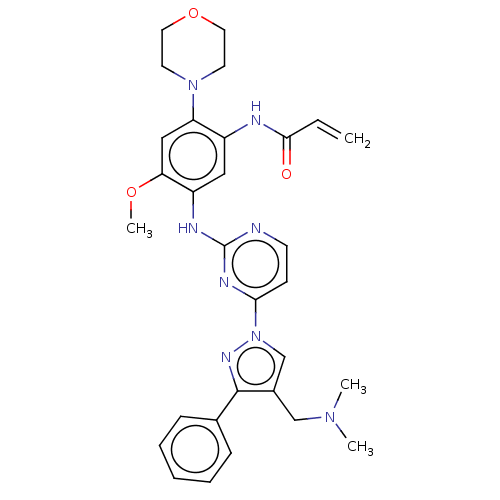
(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50402020
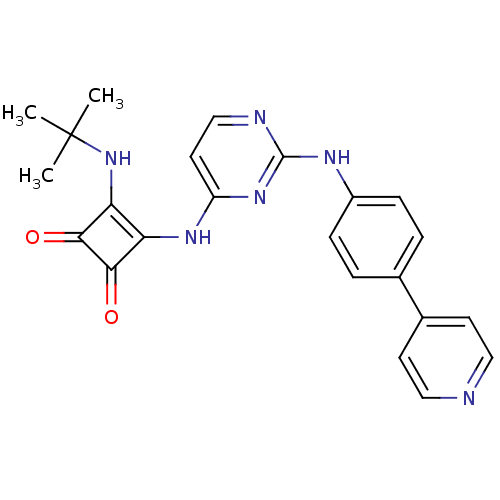
(CHEMBL2205426)Show SMILES CC(C)(C)Nc1c(Nc2ccnc(Nc3ccc(cc3)-c3ccncc3)n2)c(=O)c1=O Show InChI InChI=1S/C23H22N6O2/c1-23(2,3)29-19-18(20(30)21(19)31)27-17-10-13-25-22(28-17)26-16-6-4-14(5-7-16)15-8-11-24-12-9-15/h4-13,29H,1-3H3,(H2,25,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ERBB2 after 1 hr by scintillation counter analysis in presence of gamma-[33P]ATP |
Bioorg Med Chem Lett 22: 7615-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.009
BindingDB Entry DOI: 10.7270/Q2XK8GQ3 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50224883
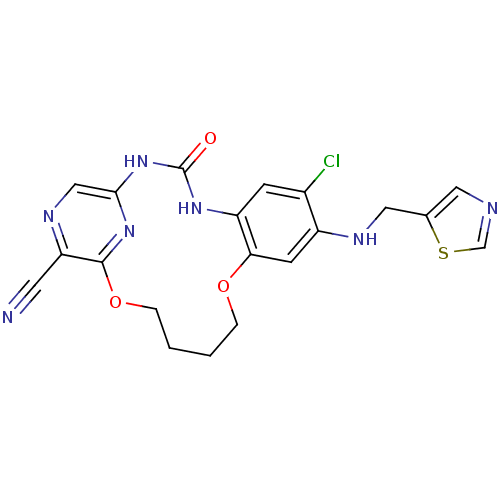
(7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,1...)Show SMILES Clc1cc2NC(=O)Nc3cnc(C#N)c(OCCCCOc2cc1NCc1cncs1)n3 Show InChI InChI=1S/C20H18ClN7O3S/c21-13-5-15-17(6-14(13)24-9-12-8-23-11-32-12)30-3-1-2-4-31-19-16(7-22)25-10-18(27-19)28-20(29)26-15/h5-6,8,10-11,24H,1-4,9H2,(H2,26,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ERBB2 |
Bioorg Med Chem Lett 17: 6593-601 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.063
BindingDB Entry DOI: 10.7270/Q2X067WT |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50390489
(CHEMBL2071602)Show SMILES CN(C)c1ccc(\C=C\c2ccc3ncnc(NCc4ccccc4)c3c2)cc1 Show InChI InChI=1S/C25H24N4/c1-29(2)22-13-10-19(11-14-22)8-9-20-12-15-24-23(16-20)25(28-18-27-24)26-17-21-6-4-3-5-7-21/h3-16,18H,17H2,1-2H3,(H,26,27,28)/b9-8+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of ERBB2 autophosphorylation Thr 1139 residue in human BT474 cells at 2 to 14 uM after 12 hrs by fluorescence assay |
Bioorg Med Chem Lett 22: 5532-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.034
BindingDB Entry DOI: 10.7270/Q2N017MV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50326046
((2S,5S,8S,11R,14R,17S)-17,21-diamino-8-(4-hydroxyb...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CCCCN)C(O)=O |r| Show InChI InChI=1S/C30H49N7O9S2/c1-16(2)11-21(30(45)46)34-27(42)22(13-38)35-26(41)20(12-17-6-8-18(39)9-7-17)33-28(43)24(15-48)37-29(44)23(14-47)36-25(40)19(32)5-3-4-10-31/h6-9,16,19-24,38-39,47-48H,3-5,10-15,31-32H2,1-2H3,(H,33,43)(H,34,42)(H,35,41)(H,36,40)(H,37,44)(H,45,46)/t19-,20-,21-,22-,23-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Molecular Biology, Medical Research Council
Curated by ChEMBL
| Assay Description
Binding affinity to ErbB-2 |
Nat Chem Biol 5: 502-7 (2009)
Article DOI: 10.1038/nchembio.184
BindingDB Entry DOI: 10.7270/Q2Z60P9P |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50395892
(CHEMBL2163772)Show InChI InChI=1S/C17H11NO3/c19-11-6-7-14-12(8-11)16-15(10-4-2-1-3-5-10)13(20)9-18-17(16)21-14/h1-9,19-20H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of ERBB2 |
Bioorg Med Chem Lett 22: 6914-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.006
BindingDB Entry DOI: 10.7270/Q2M61MC1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data