

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18070 (5-[(2-cyclopropyl-7,8-dimethoxy-2H-chromen-5-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18070 (5-[(2-cyclopropyl-7,8-dimethoxy-2H-chromen-5-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50329610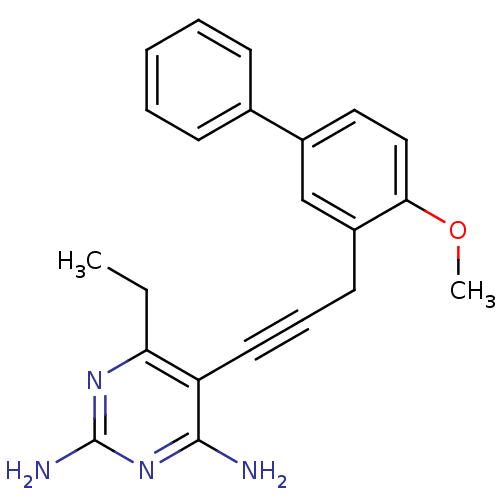 (6-ethyl-5-(3-(4-methoxybiphenyl-3-yl)prop-1-ynyl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50336514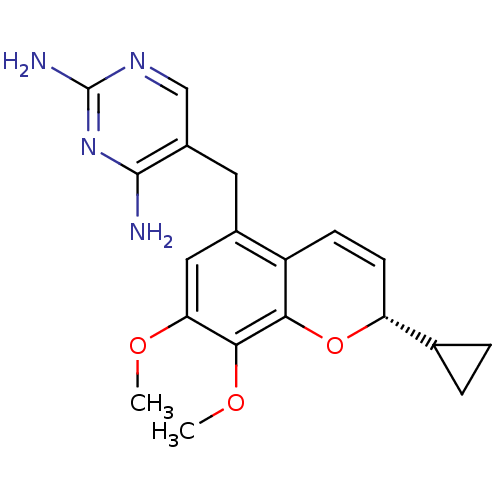 ((S)-Iclaprim | CHEMBL1673303) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50336513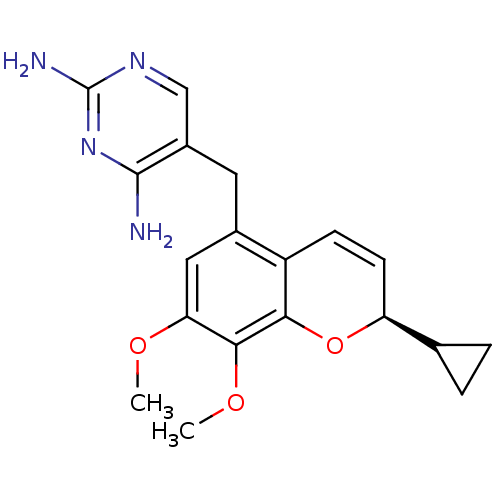 ((R)-Iclaprim | 5-[[(2R)-2-cyclopropyl-7,8-dimethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50335530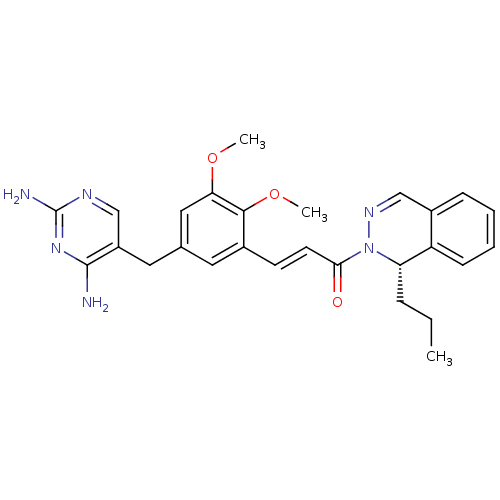 ((S)-3-(5-((2,4-diaminopyrimidin-5-yl)methyl)-2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50335530 ((S)-3-(5-((2,4-diaminopyrimidin-5-yl)methyl)-2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18070 (5-[(2-cyclopropyl-7,8-dimethoxy-2H-chromen-5-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.0 | 30 |
GSK | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Bioorg Med Chem Lett 13: 4217-21 (2003) Article DOI: 10.1016/j.bmcl.2003.07.023 BindingDB Entry DOI: 10.7270/Q2DZ06K3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.0 | 30 |
GSK | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Bioorg Med Chem Lett 13: 4217-21 (2003) Article DOI: 10.1016/j.bmcl.2003.07.023 BindingDB Entry DOI: 10.7270/Q2DZ06K3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210929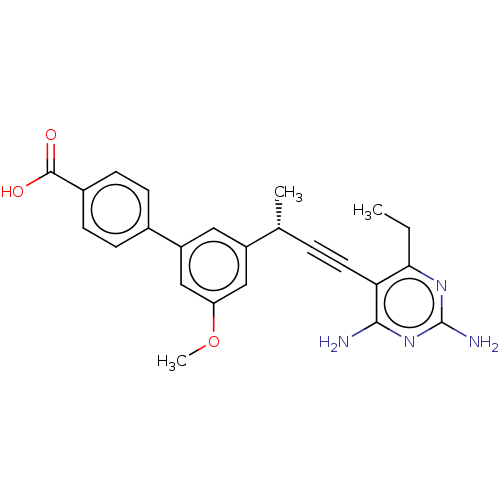 (UCP1172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut | Assay Description IC50 values were determined following a standard method that has been described previously (Reeve et al., 2014, 2016). | Cell Chem Biol 23: 1458-1467 (2016) Article DOI: 10.1016/j.chembiol.2016.11.007 BindingDB Entry DOI: 10.7270/Q2NP2380 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50294211 (3-(5-((2,4-diaminopyrimidin-5-yl)methyl)-3-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50294211 (3-(5-((2,4-diaminopyrimidin-5-yl)methyl)-3-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210932 (UCP1191) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut | Assay Description IC50 values were determined following a standard method that has been described previously (Reeve et al., 2014, 2016). | Cell Chem Biol 23: 1458-1467 (2016) Article DOI: 10.1016/j.chembiol.2016.11.007 BindingDB Entry DOI: 10.7270/Q2NP2380 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210931 (UCP1175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut | Assay Description IC50 values were determined following a standard method that has been described previously (Reeve et al., 2014, 2016). | Cell Chem Biol 23: 1458-1467 (2016) Article DOI: 10.1016/j.chembiol.2016.11.007 BindingDB Entry DOI: 10.7270/Q2NP2380 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50336512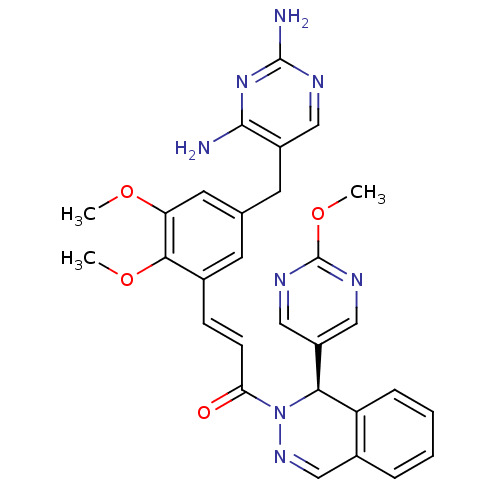 ((R)-3-(5-((2,4-diaminopyrimidin-5-yl)methyl)-2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50336512 ((R)-3-(5-((2,4-diaminopyrimidin-5-yl)methyl)-2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134287 (US8853228, 150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134287 (US8853228, 150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210930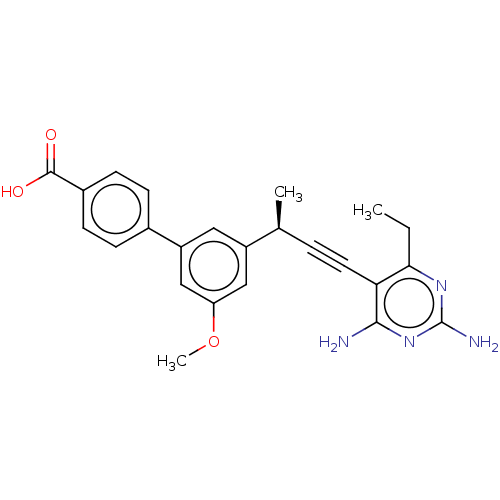 (UCP1173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut | Assay Description IC50 values were determined following a standard method that has been described previously (Reeve et al., 2014, 2016). | Cell Chem Biol 23: 1458-1467 (2016) Article DOI: 10.1016/j.chembiol.2016.11.007 BindingDB Entry DOI: 10.7270/Q2NP2380 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210927 (UCP1039) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut | Assay Description IC50 values were determined following a standard method that has been described previously (Reeve et al., 2014, 2016). | Cell Chem Biol 23: 1458-1467 (2016) Article DOI: 10.1016/j.chembiol.2016.11.007 BindingDB Entry DOI: 10.7270/Q2NP2380 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210934 (UCP1206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut | Assay Description IC50 values were determined following a standard method that has been described previously (Reeve et al., 2014, 2016). | Cell Chem Biol 23: 1458-1467 (2016) Article DOI: 10.1016/j.chembiol.2016.11.007 BindingDB Entry DOI: 10.7270/Q2NP2380 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210933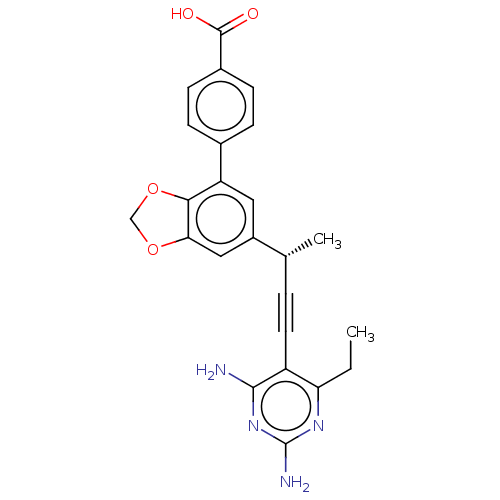 (UCP1205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut | Assay Description IC50 values were determined following a standard method that has been described previously (Reeve et al., 2014, 2016). | Cell Chem Biol 23: 1458-1467 (2016) Article DOI: 10.1016/j.chembiol.2016.11.007 BindingDB Entry DOI: 10.7270/Q2NP2380 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429697 (CHEMBL2335419) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429697 (CHEMBL2335419) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134288 (US8853228, 151) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134286 (US8853228, 149) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134286 (US8853228, 149) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut | Assay Description IC50 values were determined following a standard method that has been described previously (Reeve et al., 2014, 2016). | Cell Chem Biol 23: 1458-1467 (2016) Article DOI: 10.1016/j.chembiol.2016.11.007 BindingDB Entry DOI: 10.7270/Q2NP2380 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429698 (CHEMBL2335418) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429699 (CHEMBL2335417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429698 (CHEMBL2335418) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50329609 (5-(3-(biphenyl-3-yl)prop-1-ynyl)-6-ethylpyrimidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429700 (CHEMBL2335416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50429700 (CHEMBL2335416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134289 (US8853228, 154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134290 (US8853228, 155) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210928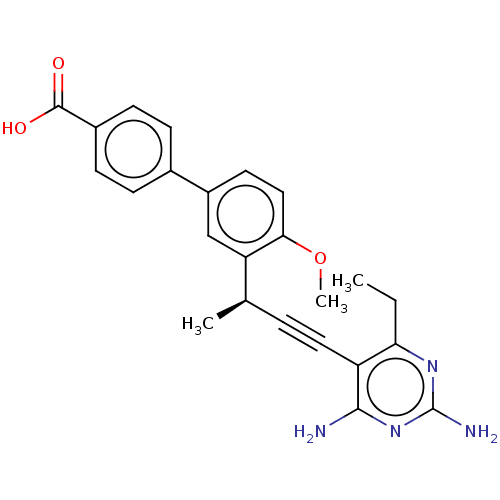 (UCP1164) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut | Assay Description IC50 values were determined following a standard method that has been described previously (Reeve et al., 2014, 2016). | Cell Chem Biol 23: 1458-1467 (2016) Article DOI: 10.1016/j.chembiol.2016.11.007 BindingDB Entry DOI: 10.7270/Q2NP2380 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50298801 ((+/-)-5-(3-(5-methoxy-3',5'-dimethylbiphenyl-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50298801 ((+/-)-5-(3-(5-methoxy-3',5'-dimethylbiphenyl-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50088488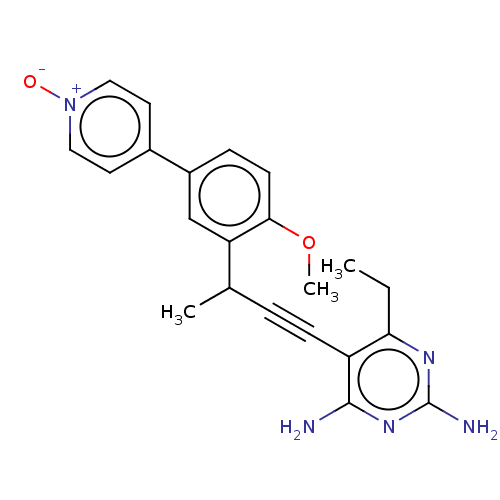 (CHEMBL3527096) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18071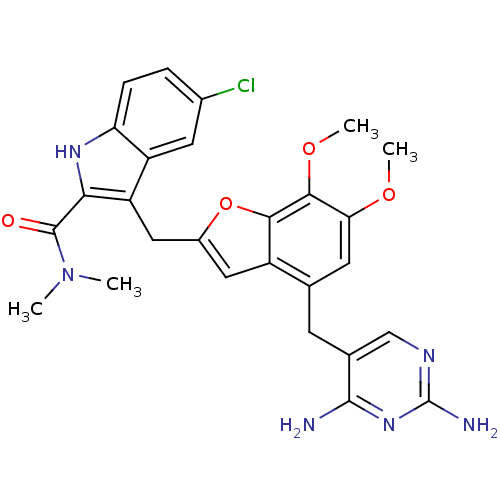 (5-chloro-3-({4-[(2,4-diaminopyrimidin-5-yl)methyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | 8 | -11.2 | 50 | n/a | n/a | n/a | n/a | 7.0 | 30 | |
ARPIDA AG | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006) BindingDB Entry DOI: 10.7270/Q2959FTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50329612 (6-ethyl-5-(3-(5-methoxybiphenyl-3-yl)prop-1-ynyl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM25826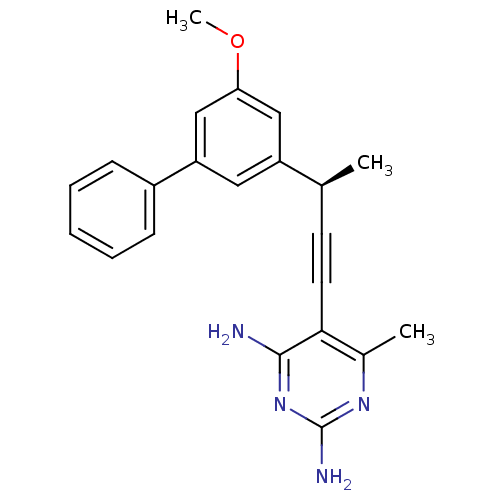 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM25818 (5-[3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]-6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50298821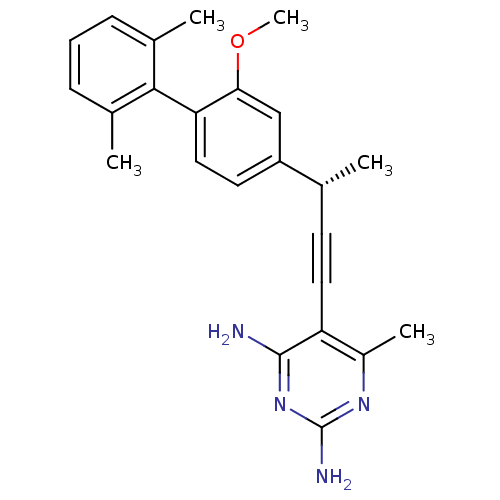 ((S)-5-(3-(2-methoxy-2',6'-dimethylbiphenyl-4-yl)bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM134285 (US8853228, 146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50007817 (CHEMBL3234115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50088487 (CHEMBL3527166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as reduction in rate of NADPH consumption | Drug Metab Dispos 40: 2002-8 (2012) Article DOI: 10.1124/dmd.112.046870 BindingDB Entry DOI: 10.7270/Q22R3TCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50298800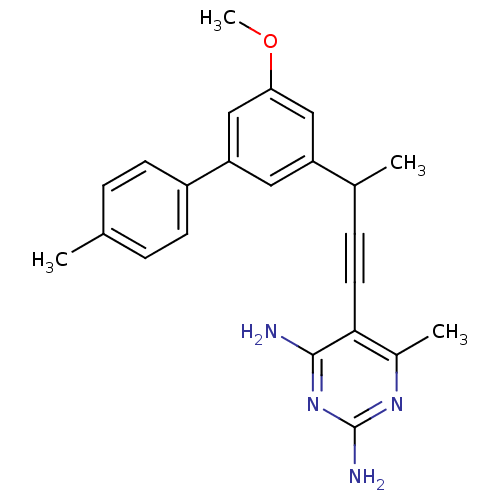 ((+/-)-5-(3-(5-methoxy-4'-methylbiphenyl-3-yl)but-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Heterocyclic analogs of propargyl-linked inhibitors of the third generation analogs were evaluated in enzyme inhibition assays, assessed for S. aur... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |