

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50598382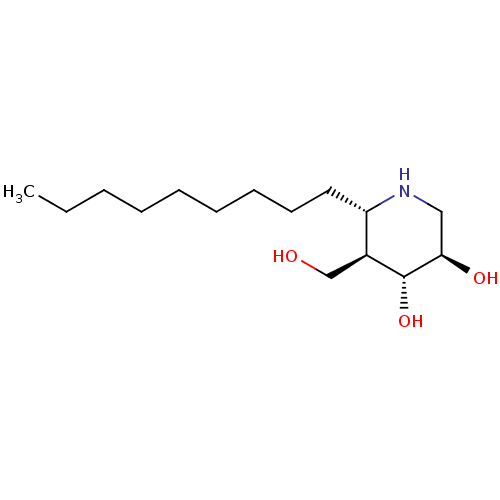 (CHEMBL5192083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114499 BindingDB Entry DOI: 10.7270/Q2Z89HFH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM36514 (CID46912122 | MDW933, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -11.6 | 1.24 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University | Assay Description Enzymatic activity assay using fluorescent activity-based labeling method. | Nat Chem Biol 6: 907-13 (2010) Article DOI: 10.1038/nchembio.466 BindingDB Entry DOI: 10.7270/Q2FX77ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM36515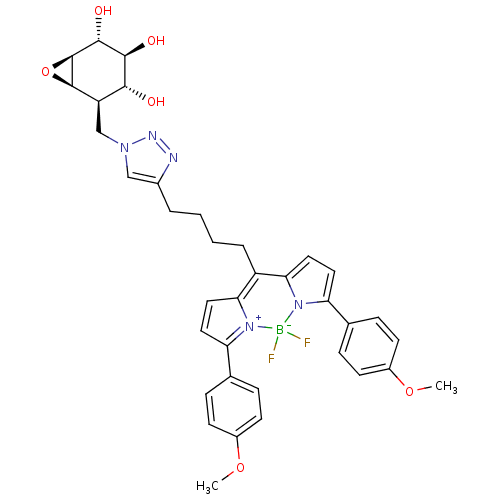 (CID46912120 | MDW941, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -11.5 | 1.94 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University | Assay Description Enzymatic activity assay using fluorescent activity-based labeling method. | Nat Chem Biol 6: 907-13 (2010) Article DOI: 10.1038/nchembio.466 BindingDB Entry DOI: 10.7270/Q2FX77ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM313450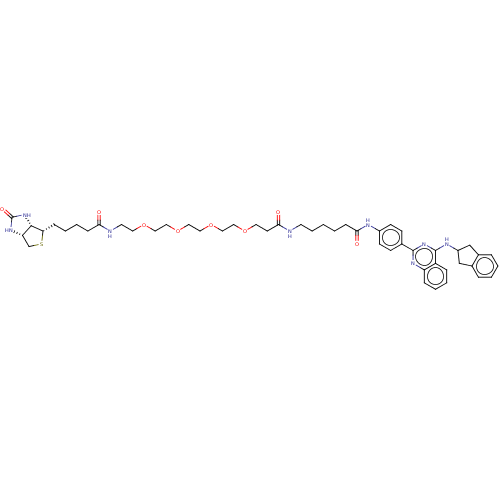 (US10167270, Example 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co... | US Patent US10167270 (2019) BindingDB Entry DOI: 10.7270/Q2862JH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM162702 (US9056847, Fluorophore 1-cyclophellitol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.950 | n/a | 2 | n/a | n/a | n/a | n/a | 5.2 | n/a |
ACADEMISCH MEDISCH CENTRUM BIJ UNIVERSITEIT VAN AMSTERDAM US Patent | Assay Description Activity of GBA was measured at 37° C. with 4-methylumbelliferyl β-D-glucopyranoside as substrate as reported previously. To determine the IC50 ... | US Patent US9056847 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108216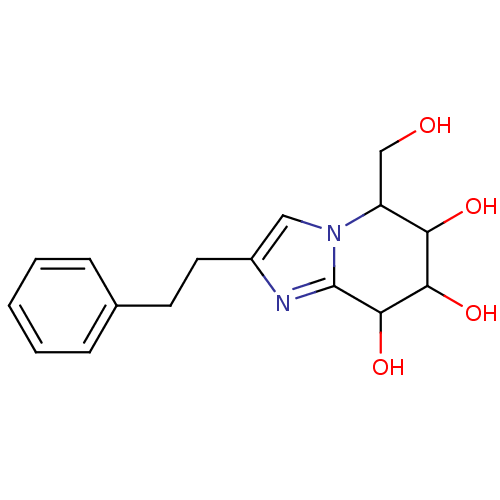 (5-(hydroxymethyl)-2-(2-phenylethyl)-5H,6H,7H,8H- i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285840 ((6R,7R,8S)-8-(but-1-yn-1-yl)-4-azaspiro[2.5]octane...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285840 ((6R,7R,8S)-8-(but-1-yn-1-yl)-4-azaspiro[2.5]octane...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285845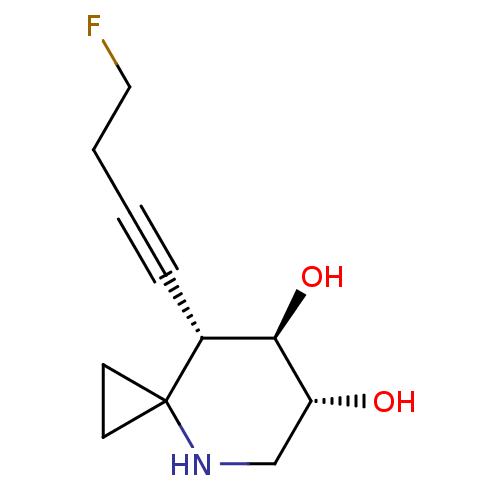 ((6R,7R,8S)-8-(4-fluorobut-1-yn-1-yl)-4-azaspiro[2....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285845 ((6R,7R,8S)-8-(4-fluorobut-1-yn-1-yl)-4-azaspiro[2....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108220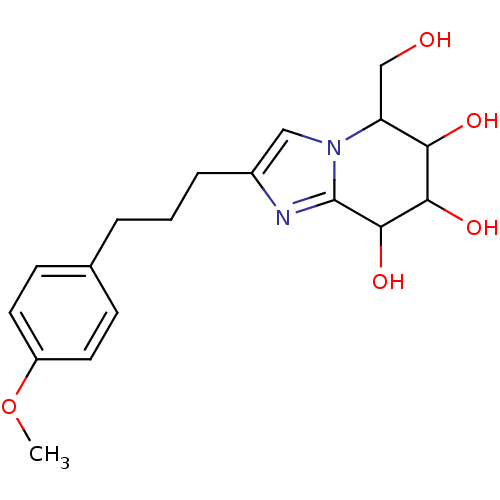 (5-(hydroxymethyl)-2-[3-(4-methoxyphenyl)propyl]- 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108220 (5-(hydroxymethyl)-2-[3-(4-methoxyphenyl)propyl]- 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.62 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285819 ((6R,7R,8S)-8-methyl-4-azaspiro[2.5]octane-6,7-diol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285819 ((6R,7R,8S)-8-methyl-4-azaspiro[2.5]octane-6,7-diol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108216 (5-(hydroxymethyl)-2-(2-phenylethyl)-5H,6H,7H,8H- i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.71 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50394827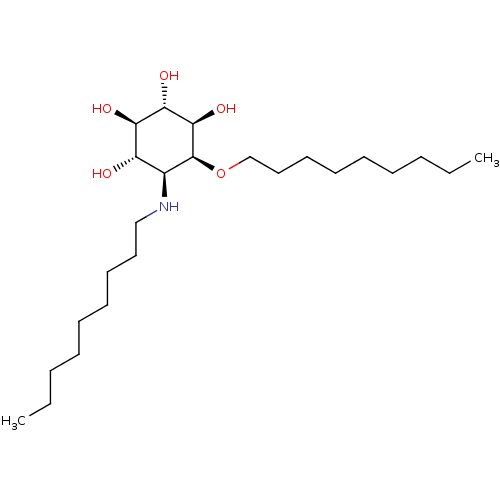 (CHEMBL2164231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before sub... | J Med Chem 55: 4479-88 (2012) Article DOI: 10.1021/jm300342q BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50394827 (CHEMBL2164231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before sub... | J Med Chem 55: 4479-88 (2012) Article DOI: 10.1021/jm300342q BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108217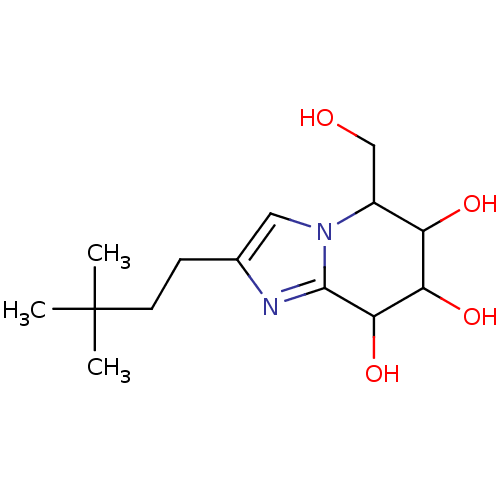 (2-(3,3-dimethylbutyl)-5-(hydroxymethyl)- 5H,6H,7H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.26 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50394827 (CHEMBL2164231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of lysosomal beta-glucosidase in human fibroblast using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 24 hrs b... | J Med Chem 55: 4479-88 (2012) Article DOI: 10.1021/jm300342q BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108219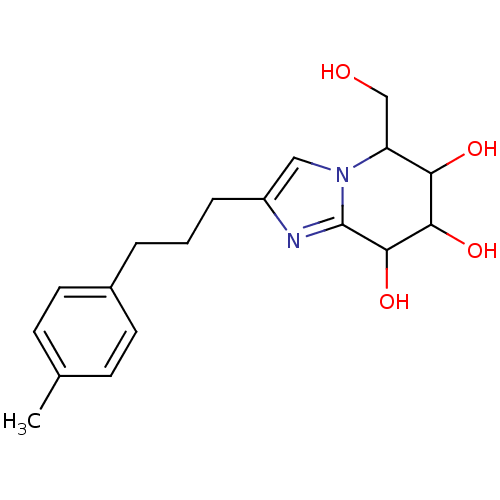 (5-(hydroxymethyl)-2-[3-(4-methylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.73 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108223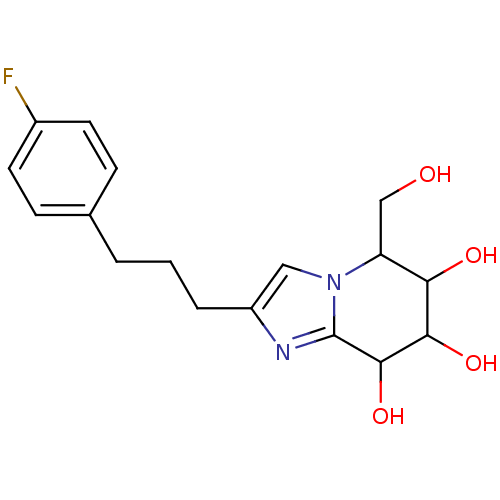 (2-[3-(4-fluorophenyl)propyl]-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.75 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50394827 (CHEMBL2164231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before sub... | J Med Chem 55: 4479-88 (2012) Article DOI: 10.1021/jm300342q BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50394827 (CHEMBL2164231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before sub... | J Med Chem 55: 4479-88 (2012) Article DOI: 10.1021/jm300342q BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108218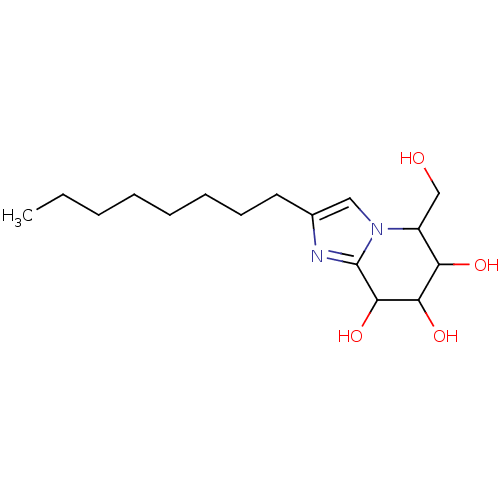 (5-(hydroxymethyl)-2-octyl-5H,6H,7H,8H-imidazo[1,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108221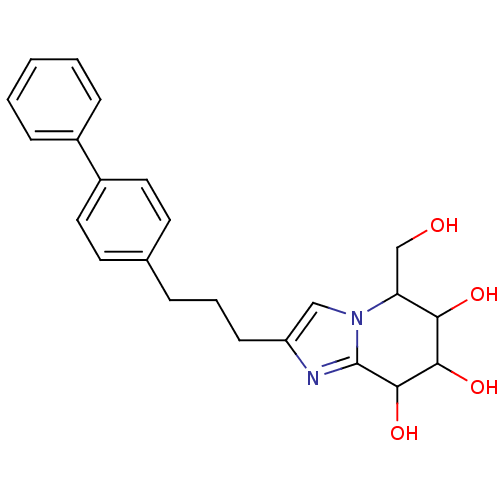 (5-(hydroxymethyl)-2-[3-(4-phenylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.11 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196623 (CHEMBL3976182 | US10167270, Example 00164 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24... | US Patent US10501435 (2019) BindingDB Entry DOI: 10.7270/Q24170FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196623 (CHEMBL3976182 | US10167270, Example 00164 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description pH = 5.9. | US Patent US10167270 (2019) BindingDB Entry DOI: 10.7270/Q2862JH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196623 (CHEMBL3976182 | US10167270, Example 00164 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co... | US Patent US10167270 (2019) BindingDB Entry DOI: 10.7270/Q2862JH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196623 (CHEMBL3976182 | US10167270, Example 00164 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 5.9 | n/a |
Northwestern University Feinberg School of Medicine Curated by ChEMBL | Assay Description Inhibition of synthetic recombinant wild type GCase enzyme velaglucerase alfa (unknown origin) at pH 5.9 preincubated for 5 mins followed by addition... | J Med Chem 59: 8508-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00930 BindingDB Entry DOI: 10.7270/Q2QF8VV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108221 (5-(hydroxymethyl)-2-[3-(4-phenylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.27 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108217 (2-(3,3-dimethylbutyl)-5-(hydroxymethyl)- 5H,6H,7H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.34 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108219 (5-(hydroxymethyl)-2-[3-(4-methylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.44 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM313446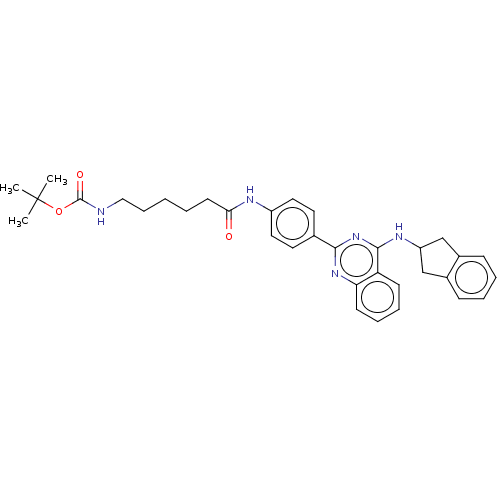 (US10167270, Example 145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co... | US Patent US10167270 (2019) BindingDB Entry DOI: 10.7270/Q2862JH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108417 (US8604206, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.06 | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amicus Therapeutics, Inc. US Patent | Assay Description The enzyme inhibition assays used monitored the ability of a test compound to bind and prevent the hydrolysis of a fluorogenic substrate in a concent... | US Patent US8604206 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM313288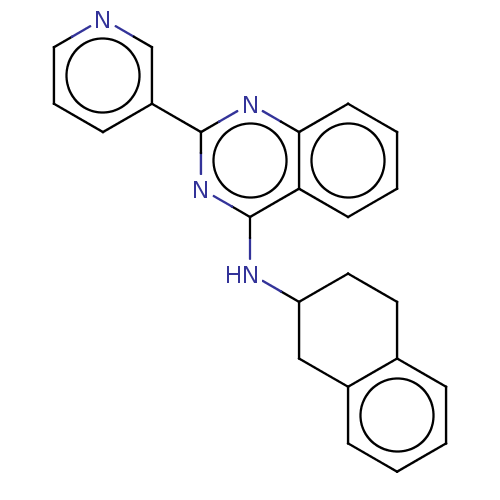 (US10167270, Example 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co... | US Patent US10167270 (2019) BindingDB Entry DOI: 10.7270/Q2862JH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196514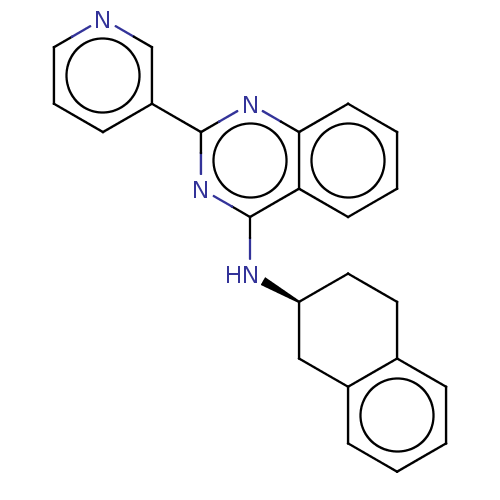 (CHEMBL3950881 | US10167270, Example 00159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co... | US Patent US10167270 (2019) BindingDB Entry DOI: 10.7270/Q2862JH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285821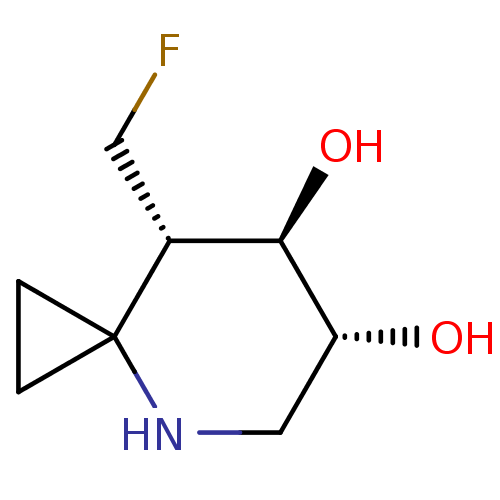 ((6R,7R,8S)-8-(fluoromethyl)-4-azaspiro[2.5]octane-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285821 ((6R,7R,8S)-8-(fluoromethyl)-4-azaspiro[2.5]octane-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108223 (2-[3-(4-fluorophenyl)propyl]-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.23 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285830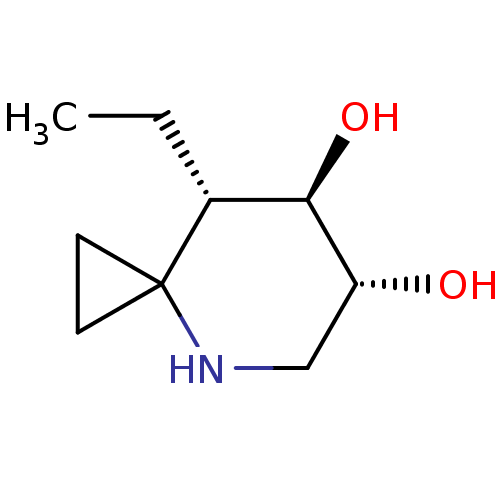 ((6R,7R,8S)-8-ethyl-4-azaspiro[2.5]octane-6,7-diol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285830 ((6R,7R,8S)-8-ethyl-4-azaspiro[2.5]octane-6,7-diol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196521 (CHEMBL3939100 | US10167270, Example 00162 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description pH = 5.9. | US Patent US10167270 (2019) BindingDB Entry DOI: 10.7270/Q2862JH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196521 (CHEMBL3939100 | US10167270, Example 00162 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The enzyme in GCase enzyme activity buffer (25 μL/well) was added to a 384-well black plate. The fluorescent probe 3 (25 nL/well, 50 nM final co... | US Patent US10167270 (2019) BindingDB Entry DOI: 10.7270/Q2862JH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196521 (CHEMBL3939100 | US10167270, Example 00162 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 5.9 | n/a |
Northwestern University Feinberg School of Medicine Curated by ChEMBL | Assay Description Inhibition of synthetic recombinant wild type GCase enzyme velaglucerase alfa (unknown origin) at pH 5.9 preincubated for 5 mins followed by addition... | J Med Chem 59: 8508-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00930 BindingDB Entry DOI: 10.7270/Q2QF8VV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196521 (CHEMBL3939100 | US10167270, Example 00162 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24... | US Patent US10501435 (2019) BindingDB Entry DOI: 10.7270/Q24170FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285822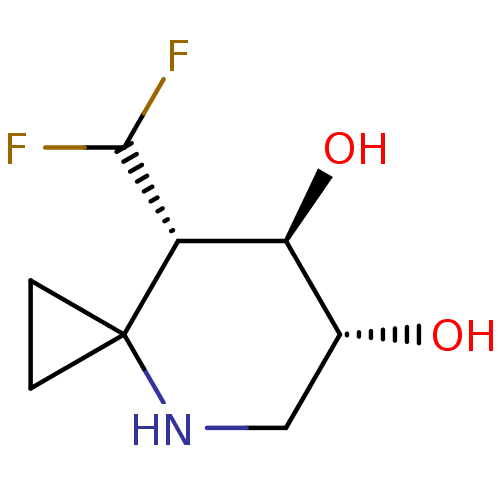 ((6R,7R,8S)-8-(difluoromethyl)-4-azaspiro[2.5]octan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285822 ((6R,7R,8S)-8-(difluoromethyl)-4-azaspiro[2.5]octan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196623 (CHEMBL3976182 | US10167270, Example 00164 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24... | US Patent US10501435 (2019) BindingDB Entry DOI: 10.7270/Q24170FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196623 (CHEMBL3976182 | US10167270, Example 00164 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University Feinberg School of Medicine Curated by ChEMBL | Assay Description Inhibition of synthetic recombinant wild type GCase enzyme velaglucerase alfa (unknown origin) at pH 7 preincubated for 5 mins followed by addition o... | J Med Chem 59: 8508-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00930 BindingDB Entry DOI: 10.7270/Q2QF8VV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196623 (CHEMBL3976182 | US10167270, Example 00164 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description pH = 7.0. | US Patent US10167270 (2019) BindingDB Entry DOI: 10.7270/Q2862JH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 895 total ) | Next | Last >> |