 Found 34 hits of kd data for polymerid = 1924
Found 34 hits of kd data for polymerid = 1924 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00716
BindingDB Entry DOI: 10.7270/Q2J1076H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM8885
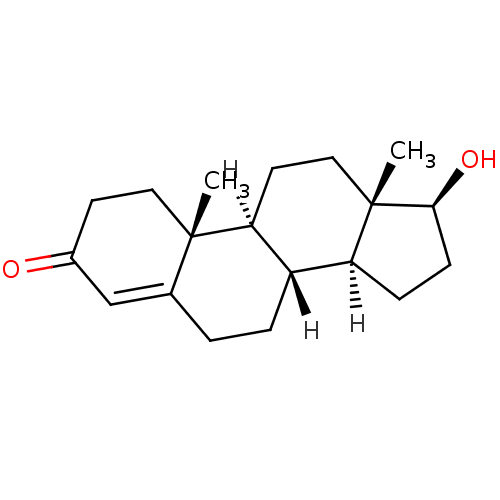
((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00716
BindingDB Entry DOI: 10.7270/Q2J1076H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Dissociation constant for [3H]R-1881 binding to human androgen receptor at 278 K |
J Med Chem 48: 2026-35 (2005)
Article DOI: 10.1021/jm040842z
BindingDB Entry DOI: 10.7270/Q2J67HQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Dissociation constant for [3H]R-1881 binding to human androgen receptor at 283 K |
J Med Chem 48: 2026-35 (2005)
Article DOI: 10.1021/jm040842z
BindingDB Entry DOI: 10.7270/Q2J67HQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Binding affinity to androgen receptor |
J Med Chem 53: 3349-60 (2010)
Article DOI: 10.1021/jm100052k
BindingDB Entry DOI: 10.7270/Q2BP03RQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Dissociation constant for [3H]R-1881 binding to human androgen receptor at 273 K |
J Med Chem 48: 2026-35 (2005)
Article DOI: 10.1021/jm040842z
BindingDB Entry DOI: 10.7270/Q2J67HQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Dissociation constant for [3H]R-1881 binding to human androgen receptor at 288 K |
J Med Chem 48: 2026-35 (2005)
Article DOI: 10.1021/jm040842z
BindingDB Entry DOI: 10.7270/Q2J67HQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Dissociation constant value of the radiolabeled compound against the androgen receptor 293 K |
J Med Chem 48: 2026-35 (2005)
Article DOI: 10.1021/jm040842z
BindingDB Entry DOI: 10.7270/Q2J67HQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Dissociation constant for [3H]R-1881 binding to human androgen receptor at 298 K |
J Med Chem 48: 2026-35 (2005)
Article DOI: 10.1021/jm040842z
BindingDB Entry DOI: 10.7270/Q2J67HQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50067678

((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...)Show SMILES C[C@H]1C[C@H]2[C@@H]3CC[C@](OC(C)=O)(C(C)=O)[C@@]3(C)CC[C@@H]2[C@@]2(C)CCC(=O)C=C12 |r,t:28| Show InChI InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3/t14-,18+,19-,20-,22+,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00716
BindingDB Entry DOI: 10.7270/Q2J1076H |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 1.21 | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Dissociation constant for [3H]R-1881 binding to human androgen receptor at 303 K |
J Med Chem 48: 2026-35 (2005)
Article DOI: 10.1021/jm040842z
BindingDB Entry DOI: 10.7270/Q2J67HQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50131272
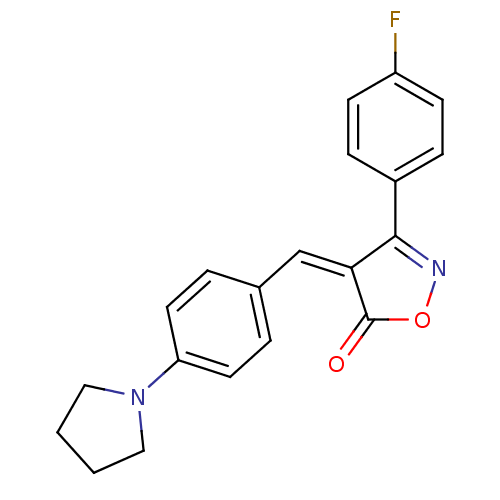
(3-(4-Fluoro-phenyl)-4-[1-(4-pyrrolidin-1-yl-phenyl...)Show SMILES Fc1ccc(cc1)C1=NOC(=O)\C1=C/c1ccc(cc1)N1CCCC1 |t:8| Show InChI InChI=1S/C20H17FN2O2/c21-16-7-5-15(6-8-16)19-18(20(24)25-22-19)13-14-3-9-17(10-4-14)23-11-1-2-12-23/h3-10,13H,1-2,11-12H2/b18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Dissociation constant against GST-hARLBD was measured in SC-3 cell by using [3H]-testosterone as radioligand |
Bioorg Med Chem Lett 13: 2655-8 (2003)
BindingDB Entry DOI: 10.7270/Q26D5SDD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50131269
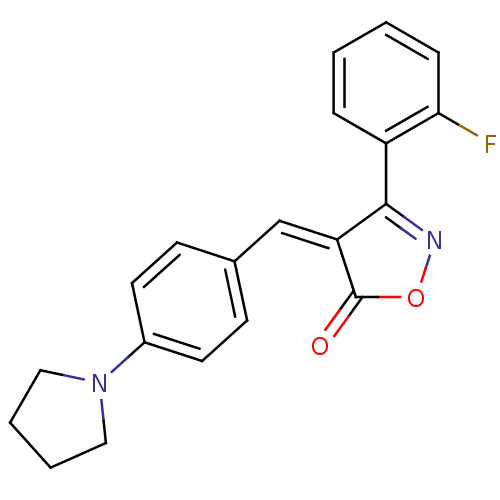
(3-(2-Fluoro-phenyl)-4-[1-(4-pyrrolidin-1-yl-phenyl...)Show SMILES Fc1ccccc1C1=NOC(=O)\C1=C/c1ccc(cc1)N1CCCC1 |t:8| Show InChI InChI=1S/C20H17FN2O2/c21-18-6-2-1-5-16(18)19-17(20(24)25-22-19)13-14-7-9-15(10-8-14)23-11-3-4-12-23/h1-2,5-10,13H,3-4,11-12H2/b17-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Dissociation constant against GST-hARLBD was measured in SC-3 cell by using [3H]-testosterone as radioligand |
Bioorg Med Chem Lett 13: 2655-8 (2003)
BindingDB Entry DOI: 10.7270/Q26D5SDD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50131268

(4-[1-(4-Dibutylamino-phenyl)-meth-(Z)-ylidene]-3-p...)Show SMILES CCCCN(CCCC)c1ccc(\C=C2/C(=O)ON=C2c2ccccc2)cc1 |c:18| Show InChI InChI=1S/C24H28N2O2/c1-3-5-16-26(17-6-4-2)21-14-12-19(13-15-21)18-22-23(25-28-24(22)27)20-10-8-7-9-11-20/h7-15,18H,3-6,16-17H2,1-2H3/b22-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Dissociation constant against GST-hARLBD was measured in SC-3 cell by using [3H]-testosterone as radioligand |
Bioorg Med Chem Lett 13: 2655-8 (2003)
BindingDB Entry DOI: 10.7270/Q26D5SDD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50131271

(3-(4-Hydroxy-phenyl)-4-[1-(4-pyrrolidin-1-yl-pheny...)Show SMILES Oc1ccc(cc1)C1=NOC(=O)\C1=C/c1ccc(cc1)N1CCCC1 |t:8| Show InChI InChI=1S/C20H18N2O3/c23-17-9-5-15(6-10-17)19-18(20(24)25-21-19)13-14-3-7-16(8-4-14)22-11-1-2-12-22/h3-10,13,23H,1-2,11-12H2/b18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Dissociation constant against GST-hARLBD was measured in SC-3 cell by using [3H]-testosterone as radioligand |
Bioorg Med Chem Lett 13: 2655-8 (2003)
BindingDB Entry DOI: 10.7270/Q26D5SDD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Dissociation constant for [3H]R-1881 binding to human androgen receptor at 308 K |
J Med Chem 48: 2026-35 (2005)
Article DOI: 10.1021/jm040842z
BindingDB Entry DOI: 10.7270/Q2J67HQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Dissociation constant for [3H]R-1881 binding to human androgen receptor at 310K |
J Med Chem 48: 2026-35 (2005)
Article DOI: 10.1021/jm040842z
BindingDB Entry DOI: 10.7270/Q2J67HQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM334273

(2-Butyl-2- hydroxy-hexanoic acid (2-bromo- pyridin...)Show InChI InChI=1S/C15H23BrN2O2/c1-3-5-8-15(20,9-6-4-2)14(19)18-12-7-10-17-13(16)11-12/h7,10-11,20H,3-6,8-9H2,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT
US Patent
| Assay Description
The N-(pyrid-4-yl)amides and N-(pyrimidin-4-yl)amides described herein exhibit androgen receptor (AR) inhibiting properties. This AR inhibiting activ... |
US Patent US9732044 (2017)
BindingDB Entry DOI: 10.7270/Q2348NHJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM334276

(2-Hydroxy-2,4- dimethyl-pentanoic acid (2-bromo-6-...)Show InChI InChI=1S/C13H19BrN2O3/c1-8(2)7-13(3,18)12(17)15-9-5-10(14)16-11(6-9)19-4/h5-6,8,18H,7H2,1-4H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT
US Patent
| Assay Description
The N-(pyrid-4-yl)amides and N-(pyrimidin-4-yl)amides described herein exhibit androgen receptor (AR) inhibiting properties. This AR inhibiting activ... |
US Patent US9732044 (2017)
BindingDB Entry DOI: 10.7270/Q2348NHJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM334272

(2-Hydroxy-2- methyl-hexanoic acid (2-bromo- 6-meth...)Show InChI InChI=1S/C13H19BrN2O3/c1-4-5-6-13(2,18)12(17)15-9-7-10(14)16-11(8-9)19-3/h7-8,18H,4-6H2,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT
US Patent
| Assay Description
The N-(pyrid-4-yl)amides and N-(pyrimidin-4-yl)amides described herein exhibit androgen receptor (AR) inhibiting properties. This AR inhibiting activ... |
US Patent US9732044 (2017)
BindingDB Entry DOI: 10.7270/Q2348NHJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM334271
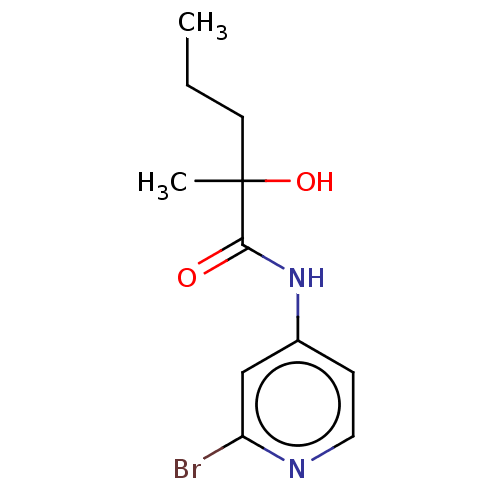
(2-Hydroxy-2- methyl- pentanoic acid (2-bromo-pyrid...)Show InChI InChI=1S/C11H15BrN2O2/c1-3-5-11(2,16)10(15)14-8-4-6-13-9(12)7-8/h4,6-7,16H,3,5H2,1-2H3,(H,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT
US Patent
| Assay Description
The N-(pyrid-4-yl)amides and N-(pyrimidin-4-yl)amides described herein exhibit androgen receptor (AR) inhibiting properties. This AR inhibiting activ... |
US Patent US9732044 (2017)
BindingDB Entry DOI: 10.7270/Q2348NHJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM334275

(N-(2-Bromo- pyridin-4-yl)-2- hydroxy-2-phenyl- pro...)Show InChI InChI=1S/C14H13BrN2O2/c1-14(19,10-5-3-2-4-6-10)13(18)17-11-7-8-16-12(15)9-11/h2-9,19H,1H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT
US Patent
| Assay Description
The N-(pyrid-4-yl)amides and N-(pyrimidin-4-yl)amides described herein exhibit androgen receptor (AR) inhibiting properties. This AR inhibiting activ... |
US Patent US9732044 (2017)
BindingDB Entry DOI: 10.7270/Q2348NHJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM334277

(N-(2-Bromo-pyridin- 4-yl)-2-hydroxy- 2-methyl-4-ph...)Show InChI InChI=1S/C16H17BrN2O2/c1-16(21,9-7-12-5-3-2-4-6-12)15(20)19-13-8-10-18-14(17)11-13/h2-6,8,10-11,21H,7,9H2,1H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT
US Patent
| Assay Description
The N-(pyrid-4-yl)amides and N-(pyrimidin-4-yl)amides described herein exhibit androgen receptor (AR) inhibiting properties. This AR inhibiting activ... |
US Patent US9732044 (2017)
BindingDB Entry DOI: 10.7270/Q2348NHJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM334274
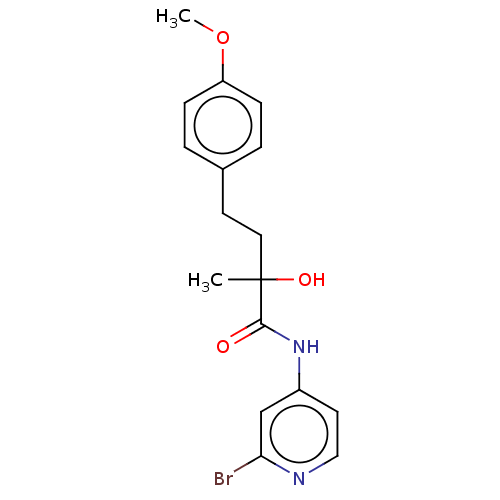
(N-(2-Bromo-pyridin- 4-yl)-2-hydroxy-4- (4-methoxy-...)Show InChI InChI=1S/C17H19BrN2O3/c1-17(22,9-7-12-3-5-14(23-2)6-4-12)16(21)20-13-8-10-19-15(18)11-13/h3-6,8,10-11,22H,7,9H2,1-2H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT
US Patent
| Assay Description
The N-(pyrid-4-yl)amides and N-(pyrimidin-4-yl)amides described herein exhibit androgen receptor (AR) inhibiting properties. This AR inhibiting activ... |
US Patent US9732044 (2017)
BindingDB Entry DOI: 10.7270/Q2348NHJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50529667

(CHEMBL4444904 | US10806720, Compound 11 | US112305...)Show SMILES C[C@](O)(Cn1ccc2cc(F)ccc12)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H15F4N3O2/c1-19(29,11-27-7-6-12-8-14(21)3-5-17(12)27)18(28)26-15-4-2-13(10-25)16(9-15)20(22,23)24/h2-9,29H,11H2,1H3,(H,26,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of androgen receptor AF1 domain (141 to 486 residues) (unknown origin) incubated for 30 mins by steady-state fluorescence emission spectro... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM334278
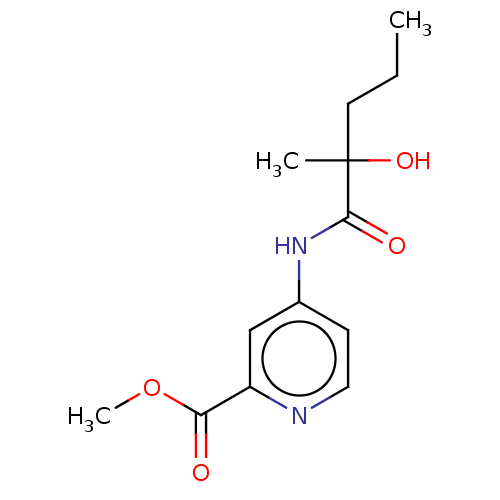
(4-(2-Hydroxy-2- methyl- pentanoylamino)- pyridine-...)Show InChI InChI=1S/C13H18N2O4/c1-4-6-13(2,18)12(17)15-9-5-7-14-10(8-9)11(16)19-3/h5,7-8,18H,4,6H2,1-3H3,(H,14,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT
US Patent
| Assay Description
The N-(pyrid-4-yl)amides and N-(pyrimidin-4-yl)amides described herein exhibit androgen receptor (AR) inhibiting properties. This AR inhibiting activ... |
US Patent US9732044 (2017)
BindingDB Entry DOI: 10.7270/Q2348NHJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50425732
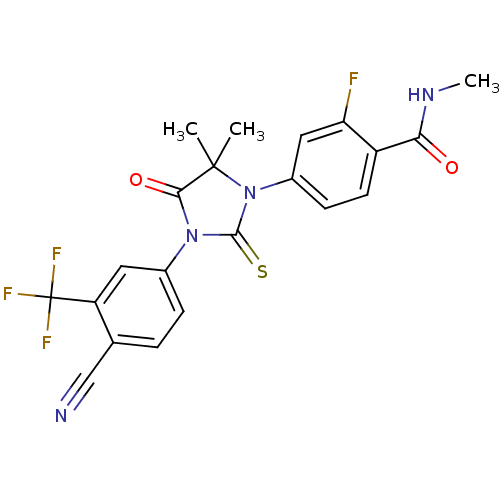
(ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...)Show SMILES CNC(=O)c1ccc(cc1F)N1C(=S)N(C(=O)C1(C)C)c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H16F4N4O2S/c1-20(2)18(31)28(12-5-4-11(10-26)15(8-12)21(23,24)25)19(32)29(20)13-6-7-14(16(22)9-13)17(30)27-3/h4-9H,1-3H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 6.52E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00912
BindingDB Entry DOI: 10.7270/Q2N58RC9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50589959

(CHEMBL5203294)Show SMILES Fc1ccc(NC(=O)Cc2ccn(n2)-c2ccc(C#N)c(c2)C(F)(F)F)cn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 8.99E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00912
BindingDB Entry DOI: 10.7270/Q2N58RC9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50589954

(CHEMBL5177079)Show SMILES Fc1ccc(NC(=O)Cc2ccn(n2)-c2ccc(C#N)c(c2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00912
BindingDB Entry DOI: 10.7270/Q2N58RC9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50589964
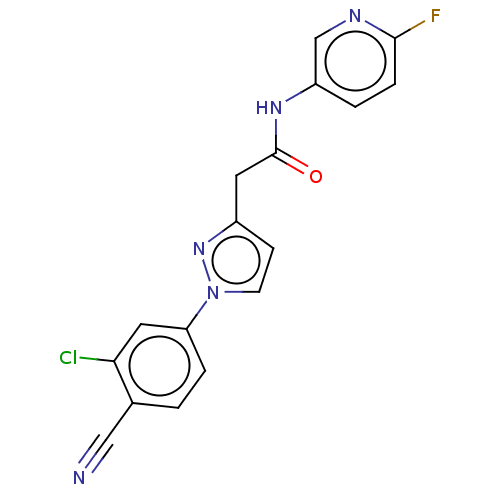
(CHEMBL5201101)Show SMILES Fc1ccc(NC(=O)Cc2ccn(n2)-c2ccc(C#N)c(Cl)c2)cn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00912
BindingDB Entry DOI: 10.7270/Q2N58RC9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50425732

(ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...)Show SMILES CNC(=O)c1ccc(cc1F)N1C(=S)N(C(=O)C1(C)C)c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H16F4N4O2S/c1-20(2)18(31)28(12-5-4-11(10-26)15(8-12)21(23,24)25)19(32)29(20)13-6-7-14(16(22)9-13)17(30)27-3/h4-9H,1-3H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 4.02E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human AR LBD (663 to 920 residues) expressed in Escherichia coli BL21 (DE3) assessed as dissociation constant by biolayer interfe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01938
BindingDB Entry DOI: 10.7270/Q27085BC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50584670
(CHEMBL5085066) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 7.59E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human AR LBD (663 to 920 residues) expressed in Escherichia coli BL21 (DE3) assessed as dissociation constant in presence of DHT ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01938
BindingDB Entry DOI: 10.7270/Q27085BC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50425732

(ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...)Show SMILES CNC(=O)c1ccc(cc1F)N1C(=S)N(C(=O)C1(C)C)c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H16F4N4O2S/c1-20(2)18(31)28(12-5-4-11(10-26)15(8-12)21(23,24)25)19(32)29(20)13-6-7-14(16(22)9-13)17(30)27-3/h4-9H,1-3H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 2.95E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human AR LBD (663 to 920 residues) expressed in Escherichia coli BL21 (DE3) assessed as dissociation constant in presence of DHT ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01938
BindingDB Entry DOI: 10.7270/Q27085BC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50584670

(CHEMBL5085066) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 7.60E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human AR LBD (663 to 920 residues) expressed in Escherichia coli BL21 (DE3) assessed as dissociation constant by biolayer interfe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01938
BindingDB Entry DOI: 10.7270/Q27085BC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data