

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50016325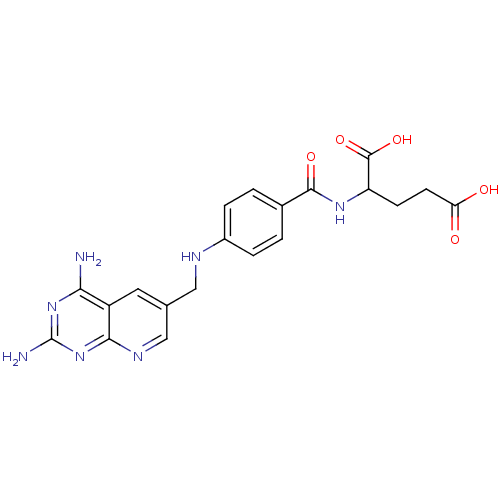 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00210 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition against Toxoplasma gondii Dihydrofolate reductase | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50035483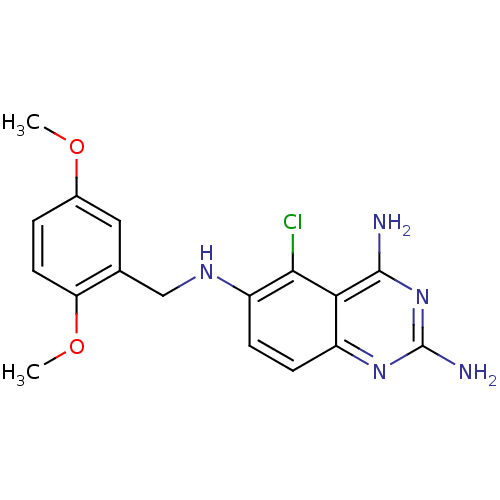 (5-Chloro-N*6*-(2,5-dimethoxy-benzyl)-quinazoline-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum DHFR | Bioorg Med Chem 25: 6467-6478 (2017) Article DOI: 10.1016/j.bmc.2017.10.017 BindingDB Entry DOI: 10.7270/Q2D50QJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18229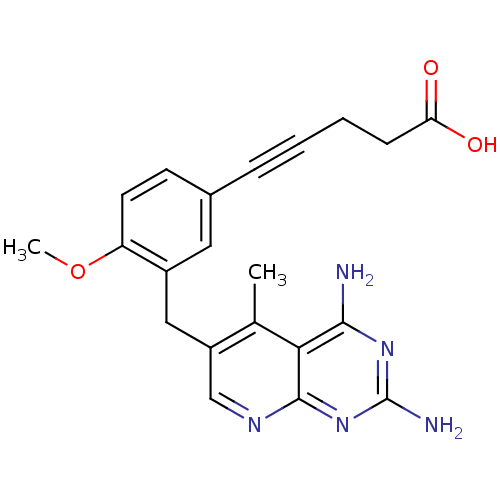 (5-[3-({2,4-diamino-5-methylpyrido[2,3-d]pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... | J Med Chem 48: 4420-31 (2005) Article DOI: 10.1021/jm0581718 BindingDB Entry DOI: 10.7270/Q2W0946F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793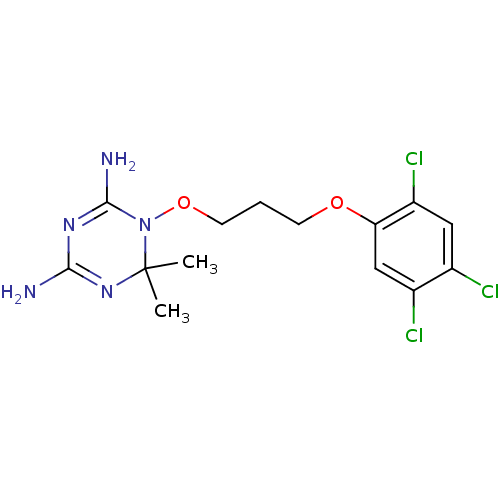 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum DHFR | Bioorg Med Chem 25: 6467-6478 (2017) Article DOI: 10.1016/j.bmc.2017.10.017 BindingDB Entry DOI: 10.7270/Q2D50QJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50029766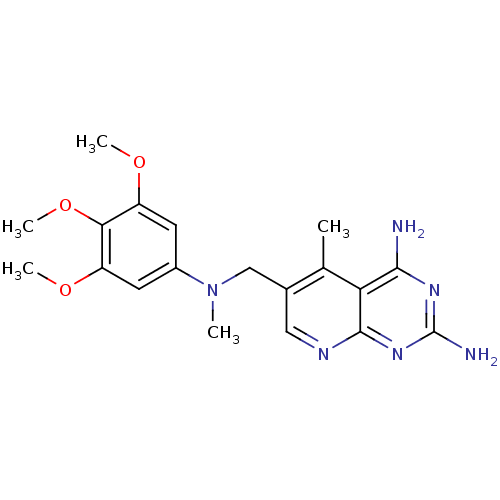 (2,4-DIAMINO-5-METHYL-6-[(3,4,5-TRIMETHOXY-N-METHYL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii dihydrofolate reductase | J Med Chem 36: 3437-43 (1993) BindingDB Entry DOI: 10.7270/Q2TH8KR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50029766 (2,4-DIAMINO-5-METHYL-6-[(3,4,5-TRIMETHOXY-N-METHYL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii dihydrofolate reductase | J Med Chem 48: 1448-69 (2005) Article DOI: 10.1021/jm040153n BindingDB Entry DOI: 10.7270/Q2V40TQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50531784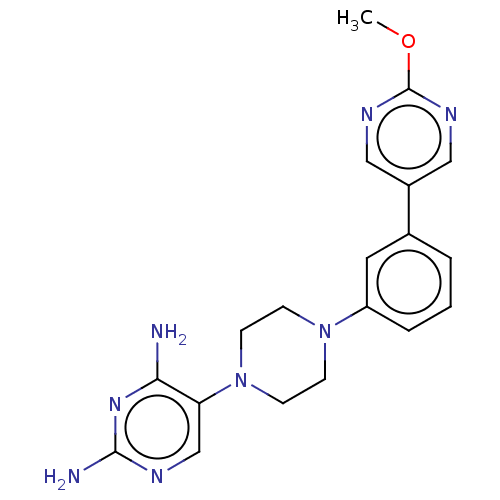 (Fanotaprim | TRC-2533 | TRC-2533-NX | US11530198, ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50049611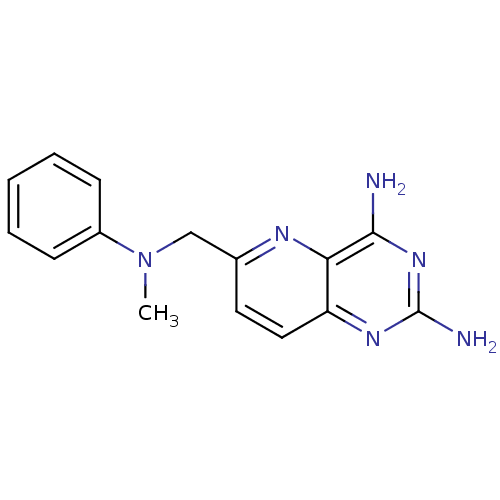 (6-[(Methyl-phenyl-amino)-methyl]-pyrido[3,2-d]pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii dihydrofolate reductase | J Med Chem 48: 1448-69 (2005) Article DOI: 10.1021/jm040153n BindingDB Entry DOI: 10.7270/Q2V40TQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50029766 (2,4-DIAMINO-5-METHYL-6-[(3,4,5-TRIMETHOXY-N-METHYL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Concentration inhibiting T. gondii dihydrofolate reductase | J Med Chem 38: 2615-20 (1995) BindingDB Entry DOI: 10.7270/Q2736PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50029766 (2,4-DIAMINO-5-METHYL-6-[(3,4,5-TRIMETHOXY-N-METHYL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of dihydrofolate reductase (DHFR) from Toxoplasma gondii(tg) | J Med Chem 42: 2447-55 (1999) Article DOI: 10.1021/jm990079m BindingDB Entry DOI: 10.7270/Q2833R62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50029766 (2,4-DIAMINO-5-METHYL-6-[(3,4,5-TRIMETHOXY-N-METHYL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase from Toxoplasma gondii | J Med Chem 38: 1778-85 (1995) BindingDB Entry DOI: 10.7270/Q2FB520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50029766 (2,4-DIAMINO-5-METHYL-6-[(3,4,5-TRIMETHOXY-N-METHYL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration against Dihydrofolate reductase from Toxoplasma gondii (tg) | J Med Chem 40: 470-8 (1997) Article DOI: 10.1021/jm9606913 BindingDB Entry DOI: 10.7270/Q26W9968 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50029766 (2,4-DIAMINO-5-METHYL-6-[(3,4,5-TRIMETHOXY-N-METHYL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration against Toxoplasma gondii Dihydrofolate reductase | J Med Chem 40: 1930-6 (1997) Article DOI: 10.1021/jm960693n BindingDB Entry DOI: 10.7270/Q2H41QHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50067865 (CHEMBL337603 | N*6*-(3,4-Dimethoxy-phenyl)-N*6*-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) from Toxoplasma gondii | J Med Chem 41: 4533-41 (1998) Article DOI: 10.1021/jm980206z BindingDB Entry DOI: 10.7270/Q2FX78KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM585318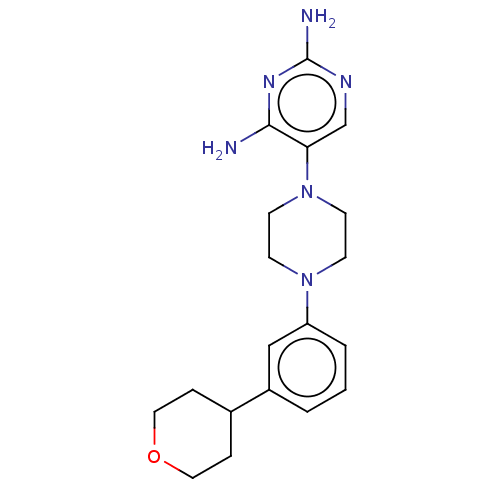 (5-(4-(3-(tetrahydro-2H-pyran-4- yl)phenyl)piperazi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase from Toxoplasma gondii was evaluated using 90 microM dihydrofolic acid as substrate | J Med Chem 40: 3040-8 (1997) Article DOI: 10.1021/jm970055k BindingDB Entry DOI: 10.7270/Q2C53JZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18497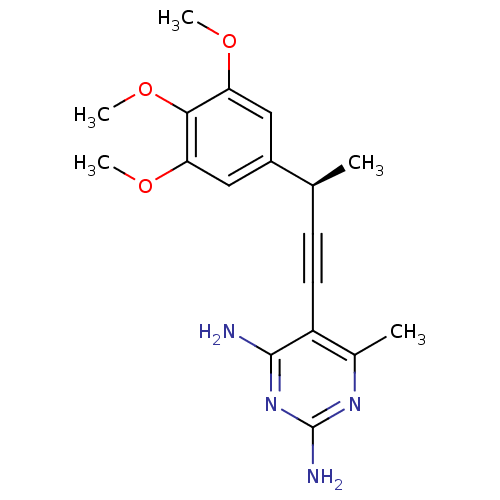 (6-methyl-5-[(3R)-3-(3,4,5-trimethoxyphenyl)but-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Dartmouth College | Assay Description Enzyme activity assays were performed by monitoring the rate of enzyme-dependent NADPH consumption at an absorbance of 340 nm over a period of severa... | J Med Chem 50: 940-50 (2007) Article DOI: 10.1021/jm061027h BindingDB Entry DOI: 10.7270/Q2KH0KKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792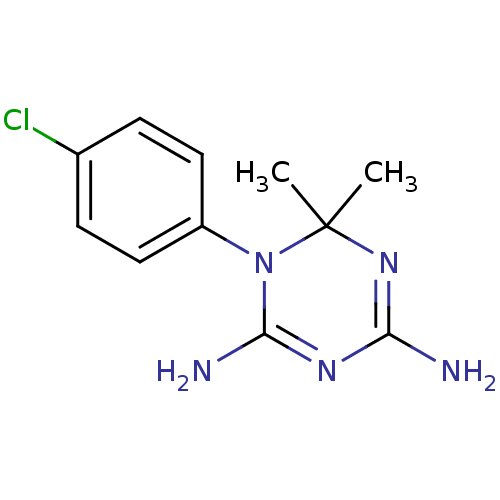 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum DHFR | Bioorg Med Chem 25: 6467-6478 (2017) Article DOI: 10.1016/j.bmc.2017.10.017 BindingDB Entry DOI: 10.7270/Q2D50QJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531784 (Fanotaprim | TRC-2533 | TRC-2533-NX | US11530198, ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50059944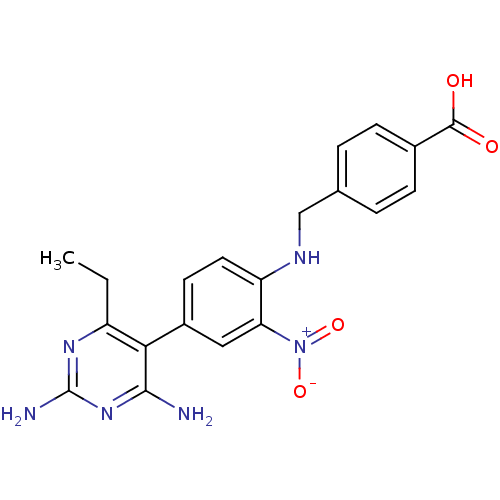 (4-{[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase from Toxoplasma gondii was evaluated using 90 microM dihydrofolic acid as substrate | J Med Chem 40: 3040-8 (1997) Article DOI: 10.1021/jm970055k BindingDB Entry DOI: 10.7270/Q2C53JZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18225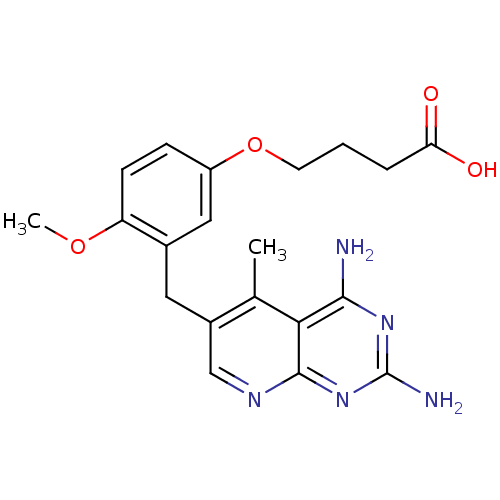 (2,4-diamino-5-deazapteridine, 4 | 4-[3-({2,4-diami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... | J Med Chem 48: 4420-31 (2005) Article DOI: 10.1021/jm0581718 BindingDB Entry DOI: 10.7270/Q2W0946F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50531779 (CHEMBL4553229 | US11530198, Example 4) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM585298 (5-(4-([1,1'-biphenyl]-3-yl)-3- methylpiperazin-1- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18227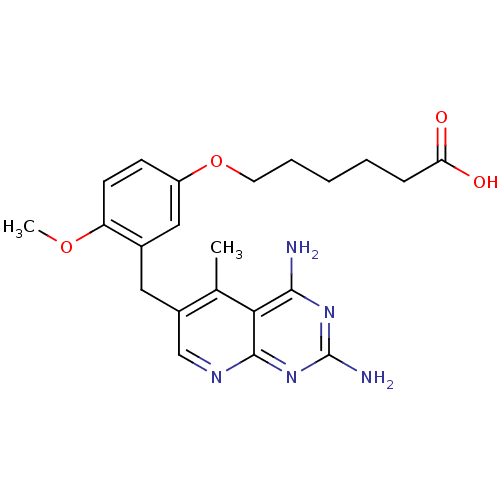 (2,4-diamino-5-deazapteridine, 6 | 6-[3-({2,4-diami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... | J Med Chem 48: 4420-31 (2005) Article DOI: 10.1021/jm0581718 BindingDB Entry DOI: 10.7270/Q2W0946F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31777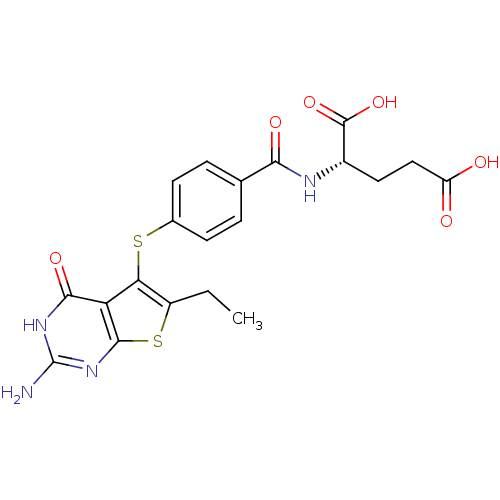 (thieno[2,3-d]pyrimidine deriv., 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50067861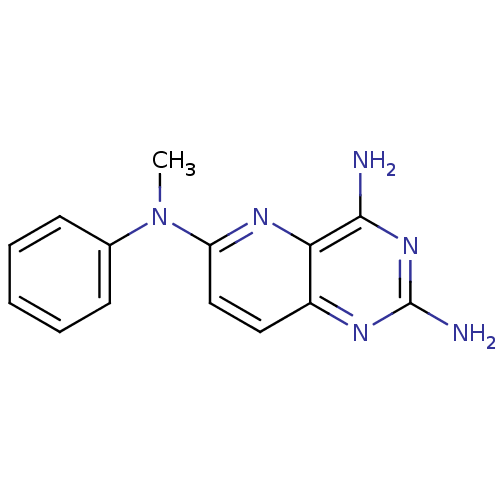 (CHEMBL140940 | N*6*-Methyl-N*6*-phenyl-pyrido[3,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) from Toxoplasma gondii | J Med Chem 41: 4533-41 (1998) Article DOI: 10.1021/jm980206z BindingDB Entry DOI: 10.7270/Q2FX78KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Leishmania major) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.0200 | -14.6 | 2.30 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18228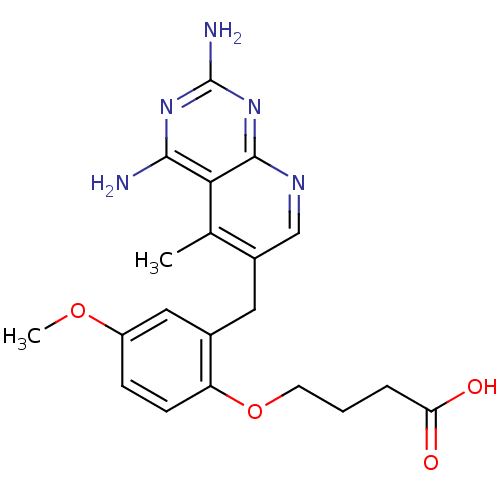 (4-[2-({2,4-diamino-5-methylpyrido[2,3-d]pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... | J Med Chem 48: 4420-31 (2005) Article DOI: 10.1021/jm0581718 BindingDB Entry DOI: 10.7270/Q2W0946F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18226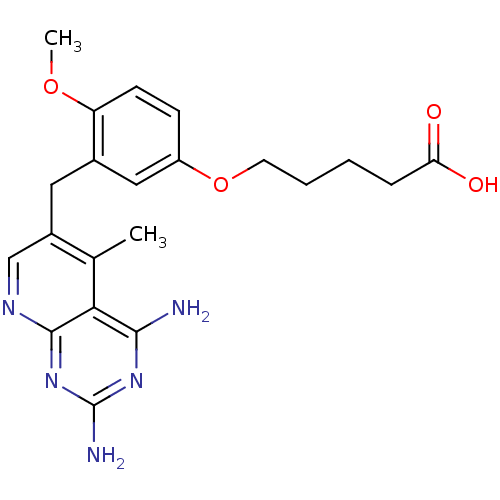 (2,4-diamino-5-deazapteridine, 5 | 5-[3-({2,4-diami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... | J Med Chem 48: 4420-31 (2005) Article DOI: 10.1021/jm0581718 BindingDB Entry DOI: 10.7270/Q2W0946F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50034942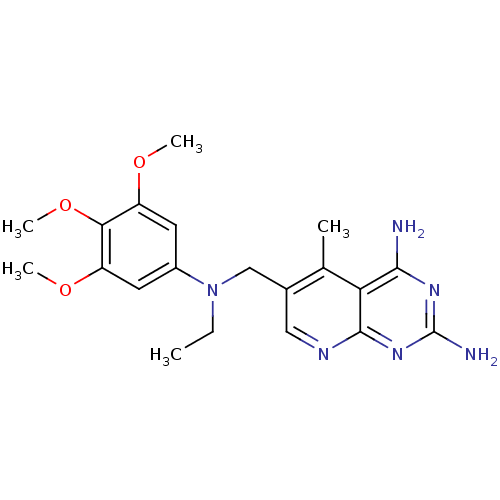 (6-{[Ethyl-(3,4,5-trimethoxy-phenyl)-amino]-methyl}...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase from Toxoplasma gondii | J Med Chem 38: 1778-85 (1995) BindingDB Entry DOI: 10.7270/Q2FB520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50034934 (6-[(3,4-Dimethoxy-phenylamino)-methyl]-5-methyl-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Toxoplasma gondii dihydrofolate reductase | J Med Chem 39: 1271-80 (1996) Article DOI: 10.1021/jm950760y BindingDB Entry DOI: 10.7270/Q2154G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531801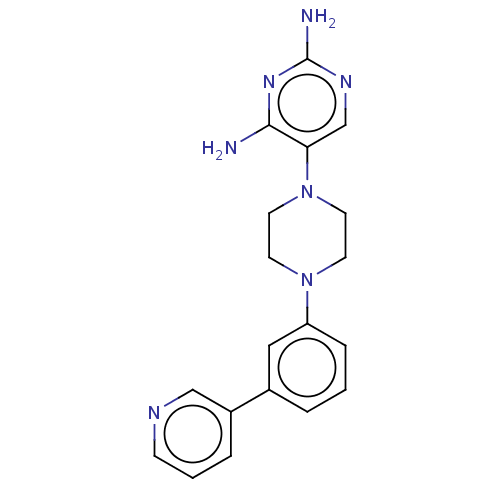 (CHEMBL4461133) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vyera Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii DHFR-TS expressed in Escherichia coli BL21 competent cells using DHF as substrate preincubated for 15 mins followed b... | J Med Chem 62: 1562-1576 (2019) Article DOI: 10.1021/acs.jmedchem.8b01754 BindingDB Entry DOI: 10.7270/Q2T43XJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50034942 (6-{[Ethyl-(3,4,5-trimethoxy-phenyl)-amino]-methyl}...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii dihydrofolate reductase | J Med Chem 48: 1448-69 (2005) Article DOI: 10.1021/jm040153n BindingDB Entry DOI: 10.7270/Q2V40TQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18784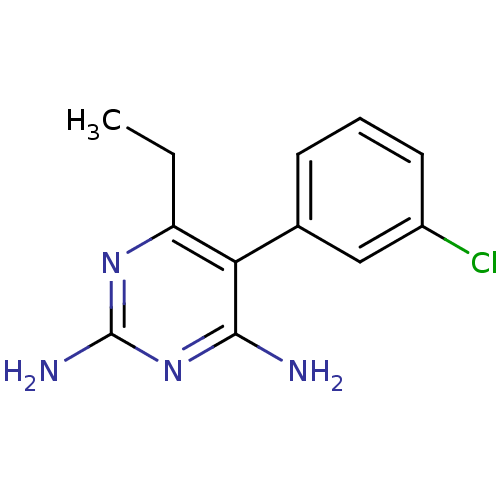 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant wild type (WT) Plasmodium falciparum DHFR-TS | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50029772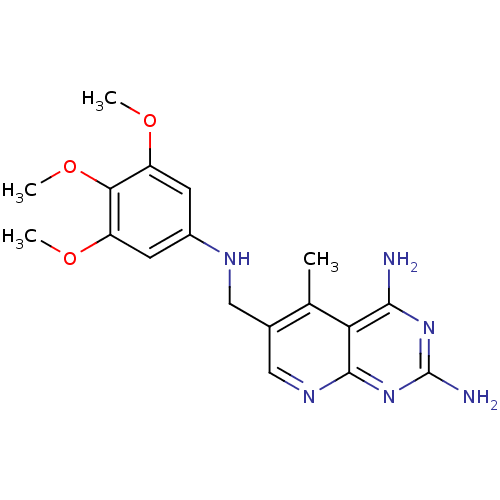 (5-Methyl-6-[(3,4,5-trimethoxy-phenylamino)-methyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Toxoplasma gondii dihydrofolate reductase | J Med Chem 39: 1271-80 (1996) Article DOI: 10.1021/jm950760y BindingDB Entry DOI: 10.7270/Q2154G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50034940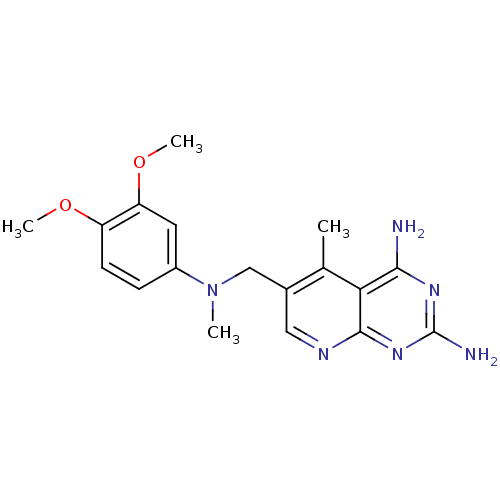 (6-{[(3,4-Dimethoxy-phenyl)-methyl-amino]-methyl}-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Toxoplasma gondii dihydrofolate reductase | J Med Chem 39: 1271-80 (1996) Article DOI: 10.1021/jm950760y BindingDB Entry DOI: 10.7270/Q2154G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50059948 (4-({[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase from Toxoplasma gondii was evaluated using 90 microM dihydrofolic acid as substrate | J Med Chem 40: 3040-8 (1997) Article DOI: 10.1021/jm970055k BindingDB Entry DOI: 10.7270/Q2C53JZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18231 (2,4-diamino-5-deazapteridine, 2 | 7-[3-({2,4-diami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... | J Med Chem 48: 4420-31 (2005) Article DOI: 10.1021/jm0581718 BindingDB Entry DOI: 10.7270/Q2W0946F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50531784 (Fanotaprim | TRC-2533 | TRC-2533-NX | US11530198, ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 3.24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18238 (2,4-diamino-5-deazapteridine, 7 | Piritrexim analo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Dihydrofolate reductase was assayed with no inhibitor and with a series of concentrations of inhibitors to allow for a range of inhibition from 10 to... | J Med Chem 48: 4420-31 (2005) Article DOI: 10.1021/jm0581718 BindingDB Entry DOI: 10.7270/Q2W0946F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM585301 (6-ethyl-5-(4-(m-tolyl)piperazin- 1-yl)pyrimidine-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50034933 (6-[(3,5-Dimethoxy-phenylamino)-methyl]-5-methyl-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Toxoplasma gondii dihydrofolate reductase | J Med Chem 39: 1271-80 (1996) Article DOI: 10.1021/jm950760y BindingDB Entry DOI: 10.7270/Q2154G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50253578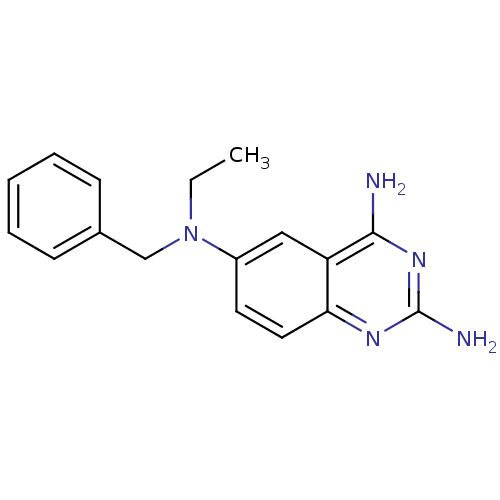 (2,4-Diamino-6-[N-(benzyl)-N-ethylamino]quinazoline...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii dihydrofolate reductase | J Med Chem 51: 6195-200 (2008) Article DOI: 10.1021/jm800694g BindingDB Entry DOI: 10.7270/Q2BP03PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50253581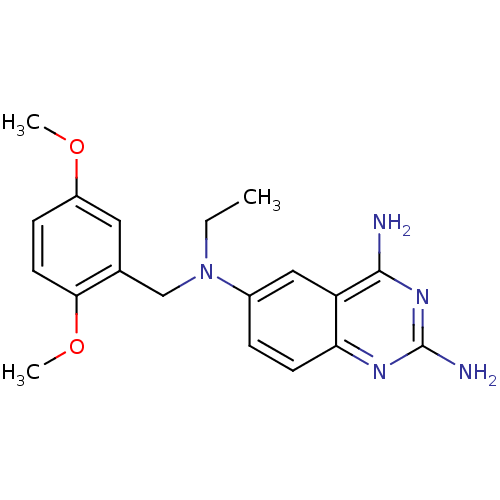 (2,4-Diamino-6-[N-(2,5-dimethoxybenzyl)-N-ethylamin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii dihydrofolate reductase | J Med Chem 51: 6195-200 (2008) Article DOI: 10.1021/jm800694g BindingDB Entry DOI: 10.7270/Q2BP03PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50059953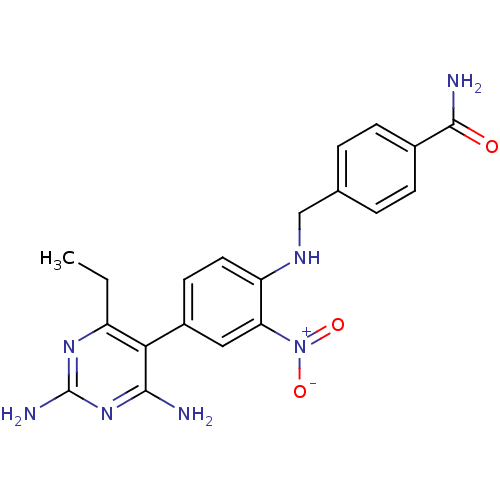 (4-{[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase from Toxoplasma gondii was evaluated using 90 microM dihydrofolic acid as substrate | J Med Chem 40: 3040-8 (1997) Article DOI: 10.1021/jm970055k BindingDB Entry DOI: 10.7270/Q2C53JZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Leishmania major) | BDBM585344 (5-(4-(quinolin-3-yl)piperazin- 1-yl)pyrimidine-2,4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8X0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1795 total ) | Next | Last >> |