 Found 400 hits of ic50 for UniProtKB: P10827
Found 400 hits of ic50 for UniProtKB: P10827 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18864
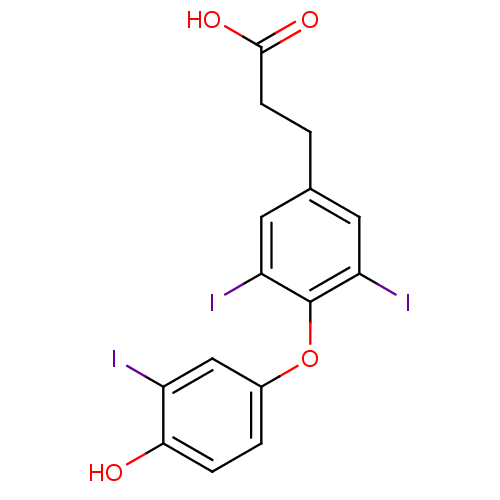
(3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]pr...)Show InChI InChI=1S/C15H11I3O4/c16-10-7-9(2-3-13(10)19)22-15-11(17)5-8(6-12(15)18)1-4-14(20)21/h2-3,5-7,19H,1,4H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0407 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18864

(3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]pr...)Show InChI InChI=1S/C15H11I3O4/c16-10-7-9(2-3-13(10)19)22-15-11(17)5-8(6-12(15)18)1-4-14(20)21/h2-3,5-7,19H,1,4H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18865
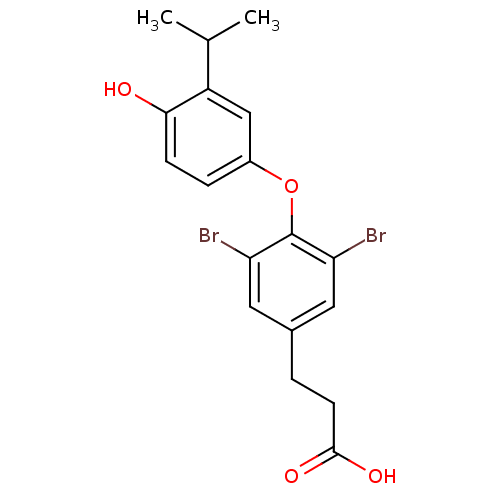
(3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C18H18Br2O4/c1-10(2)13-9-12(4-5-16(13)21)24-18-14(19)7-11(8-15(18)20)3-6-17(22)23/h4-5,7-10,21H,3,6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18865

(3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C18H18Br2O4/c1-10(2)13-9-12(4-5-16(13)21)24-18-14(19)7-11(8-15(18)20)3-6-17(22)23/h4-5,7-10,21H,3,6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18863

((2R)-2-amino-3-{4-[4-hydroxy-3-(propan-2-yl)phenox...)Show SMILES CC(C)c1cc(Oc2c(I)cc(C[C@@H](N)C(O)=O)cc2I)ccc1O |r| Show InChI InChI=1S/C18H19I2NO4/c1-9(2)12-8-11(3-4-16(12)22)25-17-13(19)5-10(6-14(17)20)7-15(21)18(23)24/h3-6,8-9,15,22H,7,21H2,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18862
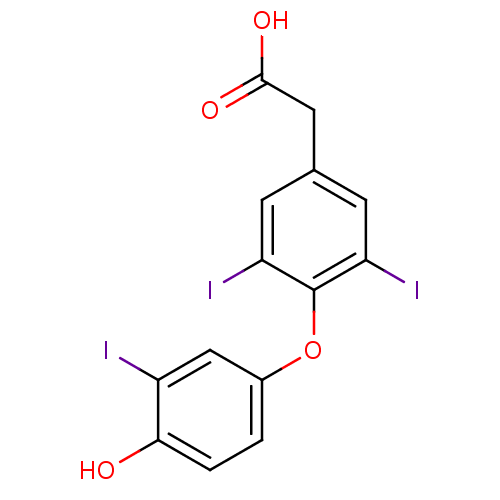
(2-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]ac...)Show InChI InChI=1S/C14H9I3O4/c15-9-6-8(1-2-12(9)18)21-14-10(16)3-7(4-11(14)17)5-13(19)20/h1-4,6,18H,5H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18863

((2R)-2-amino-3-{4-[4-hydroxy-3-(propan-2-yl)phenox...)Show SMILES CC(C)c1cc(Oc2c(I)cc(C[C@@H](N)C(O)=O)cc2I)ccc1O |r| Show InChI InChI=1S/C18H19I2NO4/c1-9(2)12-8-11(3-4-16(12)22)25-17-13(19)5-10(6-14(17)20)7-15(21)18(23)24/h3-6,8-9,15,22H,7,21H2,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.141 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18862

(2-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]ac...)Show InChI InChI=1S/C14H9I3O4/c15-9-6-8(1-2-12(9)18)21-14-10(16)3-7(4-11(14)17)5-13(19)20/h1-4,6,18H,5H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 0.141 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860
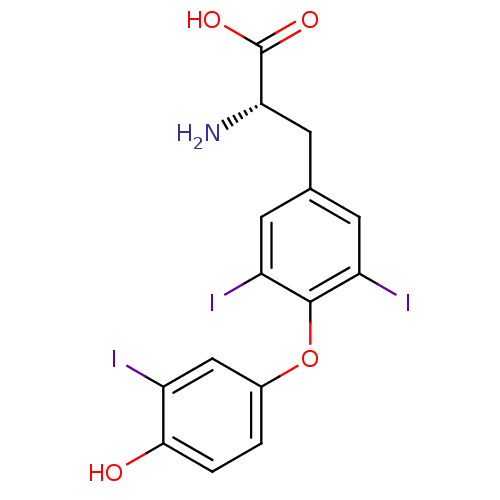
((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor alpha 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50178971
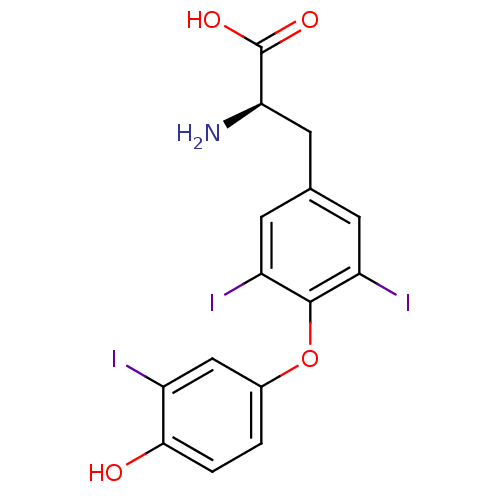
((R)-2-Amino-3-[4-(4-hydroxy-3-iodo-phenoxy)-3,5-di...)Show SMILES N[C@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB
Curated by ChEMBL
| Assay Description
Inhibition of human TRalpha1 by radioligand binding assay |
Bioorg Med Chem Lett 16: 1240-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.077
BindingDB Entry DOI: 10.7270/Q2T72H1W |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50178971

((R)-2-Amino-3-[4-(4-hydroxy-3-iodo-phenoxy)-3,5-di...)Show SMILES N[C@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 16: 884-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.002
BindingDB Entry DOI: 10.7270/Q2BV7DWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18870
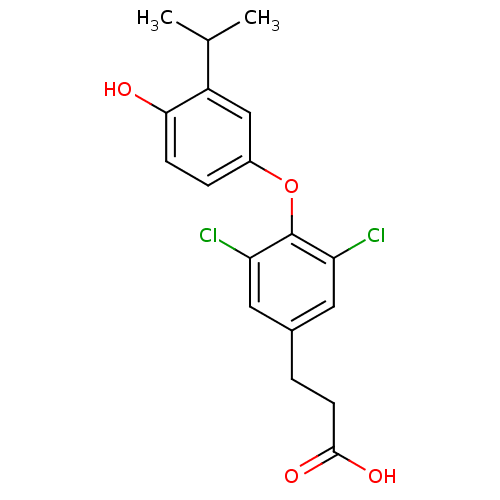
(3-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C18H18Cl2O4/c1-10(2)13-9-12(4-5-16(13)21)24-18-14(19)7-11(8-15(18)20)3-6-17(22)23/h4-5,7-10,21H,3,6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.759 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18870

(3-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C18H18Cl2O4/c1-10(2)13-9-12(4-5-16(13)21)24-18-14(19)7-11(8-15(18)20)3-6-17(22)23/h4-5,7-10,21H,3,6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.760 | n/a | 0.300 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18867
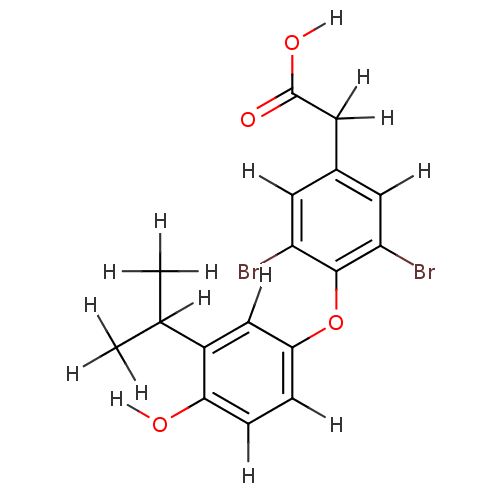
(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | 0.380 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50178975
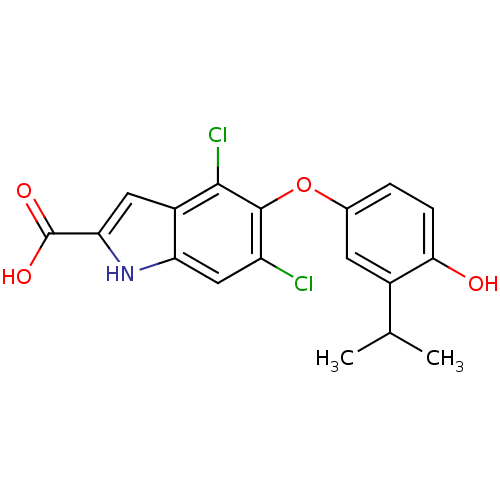
(4,6-dichloro-5-(4-hydroxy-3-isopropylphenoxy)-1H-i...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc3[nH]c(cc3c2Cl)C(O)=O)ccc1O Show InChI InChI=1S/C18H15Cl2NO4/c1-8(2)10-5-9(3-4-15(10)22)25-17-12(19)7-13-11(16(17)20)6-14(21-13)18(23)24/h3-8,21-22H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB
Curated by ChEMBL
| Assay Description
Inhibition of human TRalpha1 by radioligand binding assay |
Bioorg Med Chem Lett 16: 1240-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.077
BindingDB Entry DOI: 10.7270/Q2T72H1W |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18867

(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | 0.380 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18867

(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50178975

(4,6-dichloro-5-(4-hydroxy-3-isopropylphenoxy)-1H-i...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc3[nH]c(cc3c2Cl)C(O)=O)ccc1O Show InChI InChI=1S/C18H15Cl2NO4/c1-8(2)10-5-9(3-4-15(10)22)25-17-12(19)7-13-11(16(17)20)6-14(21-13)18(23)24/h3-8,21-22H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18867

(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50178974
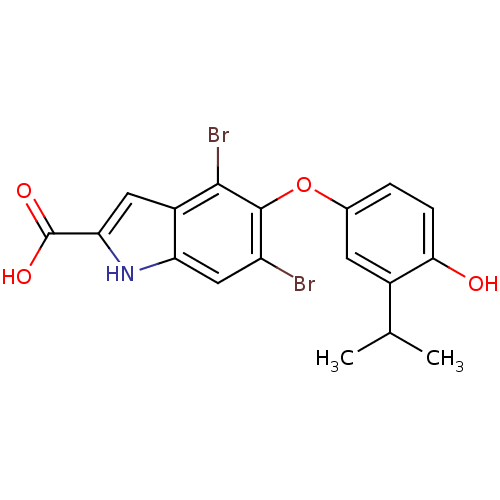
(4,6-dibromo-5-(4-hydroxy-3-isopropylphenoxy)-1H-in...)Show SMILES CC(C)c1cc(Oc2c(Br)cc3[nH]c(cc3c2Br)C(O)=O)ccc1O Show InChI InChI=1S/C18H15Br2NO4/c1-8(2)10-5-9(3-4-15(10)22)25-17-12(19)7-13-11(16(17)20)6-14(21-13)18(23)24/h3-8,21-22H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB
Curated by ChEMBL
| Assay Description
Inhibition of human TRalpha1 by radioligand binding assay |
Bioorg Med Chem Lett 16: 1240-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.077
BindingDB Entry DOI: 10.7270/Q2T72H1W |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50178974

(4,6-dibromo-5-(4-hydroxy-3-isopropylphenoxy)-1H-in...)Show SMILES CC(C)c1cc(Oc2c(Br)cc3[nH]c(cc3c2Br)C(O)=O)ccc1O Show InChI InChI=1S/C18H15Br2NO4/c1-8(2)10-5-9(3-4-15(10)22)25-17-12(19)7-13-11(16(17)20)6-14(21-13)18(23)24/h3-8,21-22H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanwa Kagaku Kenkyusho Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Triiodothyronine from human recombinant TRalpha1 ligand binding domain after 2 to 3 hrs by beta counting |
Bioorg Med Chem 22: 488-98 (2013)
Article DOI: 10.1016/j.bmc.2013.11.001
BindingDB Entry DOI: 10.7270/Q2H41SXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18874

((2S)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)[C@H](NC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(C)C)c(Br)c1)C(O)=O |r| Show InChI InChI=1S/C22H25Br2NO5/c1-11(2)15-10-14(5-6-18(15)26)30-21-16(23)7-13(8-17(21)24)9-19(27)25-20(12(3)4)22(28)29/h5-8,10-12,20,26H,9H2,1-4H3,(H,25,27)(H,28,29)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | 1.40 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50171808
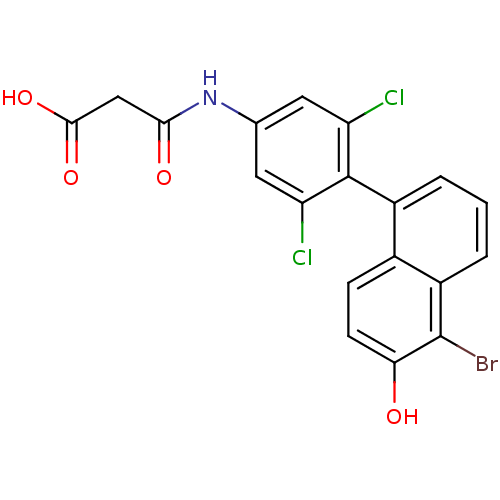
(CHEMBL194242 | N-[4-(5-Bromo-6-hydroxy-naphthalen-...)Show SMILES OC(=O)CC(=O)Nc1cc(Cl)c(c(Cl)c1)-c1cccc2c(Br)c(O)ccc12 |(13.88,-4.76,;13.87,-3.22,;15.2,-2.45,;12.54,-2.46,;12.53,-.92,;13.87,-.14,;11.2,-.15,;9.85,-.92,;9.87,-2.46,;8.52,-3.24,;8.52,-4.78,;7.19,-2.47,;7.19,-.93,;5.86,-.16,;8.52,-.16,;5.86,-3.25,;5.86,-4.78,;4.53,-5.56,;3.2,-4.79,;3.2,-3.25,;1.87,-2.48,;.52,-3.25,;1.87,-.93,;.54,-.17,;3.2,-.16,;4.53,-.93,;4.53,-2.48,)| Show InChI InChI=1S/C19H12BrCl2NO4/c20-19-12-3-1-2-11(10(12)4-5-15(19)24)18-13(21)6-9(7-14(18)22)23-16(25)8-17(26)27/h1-7,24H,8H2,(H,23,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor alpha 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50171801
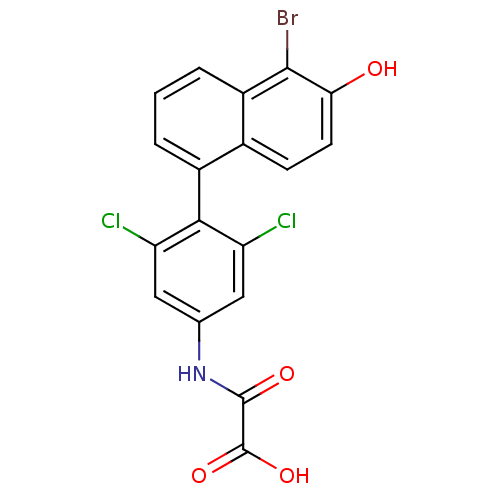
(CHEMBL198367 | N-[4-(5-Bromo-6-hydroxy-naphthalen-...)Show SMILES OC(=O)C(=O)Nc1cc(Cl)c(c(Cl)c1)-c1cccc2c(Br)c(O)ccc12 |(11.21,-4.35,;12.54,-3.57,;13.87,-4.32,;12.53,-2.03,;13.87,-1.24,;11.2,-1.26,;9.85,-2.04,;8.52,-1.27,;7.19,-2.04,;5.86,-1.27,;7.19,-3.58,;8.52,-4.35,;8.52,-5.89,;9.87,-3.58,;5.86,-4.36,;5.86,-5.89,;4.53,-6.67,;3.2,-5.9,;3.2,-4.36,;1.87,-3.59,;.52,-4.36,;1.87,-2.05,;.54,-1.28,;3.2,-1.27,;4.53,-2.04,;4.53,-3.59,)| Show InChI InChI=1S/C18H10BrCl2NO4/c19-16-11-3-1-2-10(9(11)4-5-14(16)23)15-12(20)6-8(7-13(15)21)22-17(24)18(25)26/h1-7,23H,(H,22,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor alpha 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18877
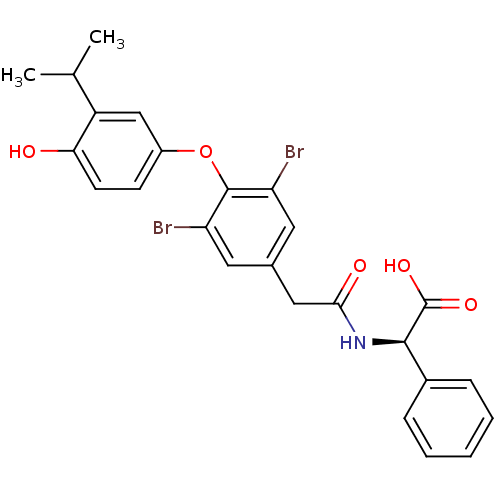
((2R)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CC(=O)N[C@@H](C(O)=O)c3ccccc3)cc2Br)ccc1O |r| Show InChI InChI=1S/C25H23Br2NO5/c1-14(2)18-13-17(8-9-21(18)29)33-24-19(26)10-15(11-20(24)27)12-22(30)28-23(25(31)32)16-6-4-3-5-7-16/h3-11,13-14,23,29H,12H2,1-2H3,(H,28,30)(H,31,32)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | 1.5 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
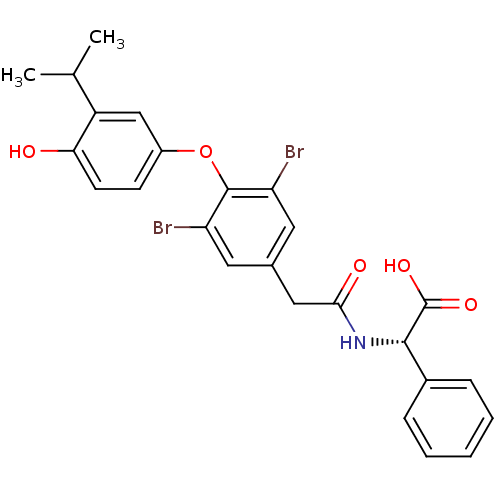
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18876
((2S)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CC(=O)N[C@H](C(O)=O)c3ccccc3)cc2Br)ccc1O |r| Show InChI InChI=1S/C25H23Br2NO5/c1-14(2)18-13-17(8-9-21(18)29)33-24-19(26)10-15(11-20(24)27)12-22(30)28-23(25(31)32)16-6-4-3-5-7-16/h3-11,13-14,23,29H,12H2,1-2H3,(H,28,30)(H,31,32)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
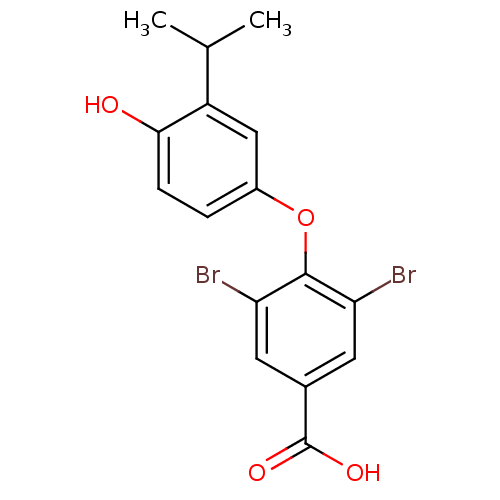
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18866
(3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy]be...)Show InChI InChI=1S/C16H14Br2O4/c1-8(2)11-7-10(3-4-14(11)19)22-15-12(17)5-9(16(20)21)6-13(15)18/h3-8,19H,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18866

(3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy]be...)Show InChI InChI=1S/C16H14Br2O4/c1-8(2)11-7-10(3-4-14(11)19)22-15-12(17)5-9(16(20)21)6-13(15)18/h3-8,19H,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
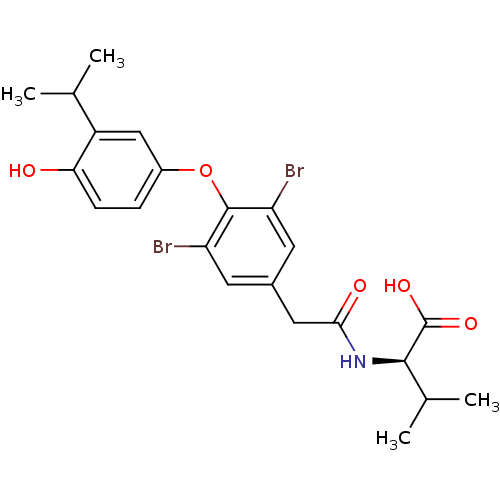
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18875
((2R)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)[C@@H](NC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(C)C)c(Br)c1)C(O)=O |r| Show InChI InChI=1S/C22H25Br2NO5/c1-11(2)15-10-14(5-6-18(15)26)30-21-16(23)7-13(8-17(21)24)9-19(27)25-20(12(3)4)22(28)29/h5-8,10-12,20,26H,9H2,1-4H3,(H,25,27)(H,28,29)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | 2.30 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
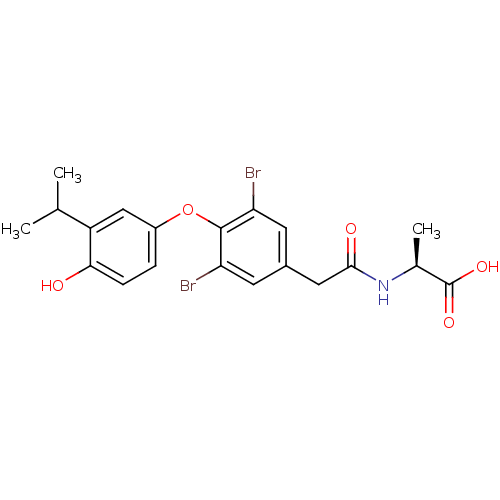
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18872
((2S)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CC(=O)N[C@@H](C)C(O)=O)cc2Br)ccc1O |r| Show InChI InChI=1S/C20H21Br2NO5/c1-10(2)14-9-13(4-5-17(14)24)28-19-15(21)6-12(7-16(19)22)8-18(25)23-11(3)20(26)27/h4-7,9-11,24H,8H2,1-3H3,(H,23,25)(H,26,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | 7.20 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18913
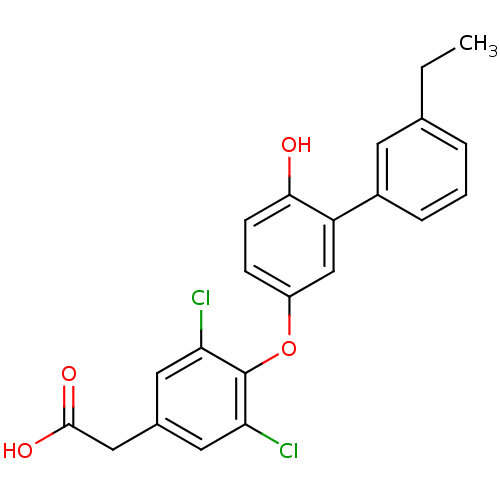
(2-{3,5-dichloro-4-[3-(3-ethylphenyl)-4-hydroxyphen...)Show SMILES CCc1cccc(c1)-c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)ccc1O Show InChI InChI=1S/C22H18Cl2O4/c1-2-13-4-3-5-15(8-13)17-12-16(6-7-20(17)25)28-22-18(23)9-14(10-19(22)24)11-21(26)27/h3-10,12,25H,2,11H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50171803

(3-[4-(5-Bromo-6-hydroxy-naphthalen-1-yl)-3,5-dichl...)Show SMILES OC(=O)CCNc1cc(Cl)c(c(Cl)c1)-c1cccc2c(Br)c(O)ccc12 |(13.88,-5.86,;13.87,-4.32,;15.2,-3.55,;12.54,-3.57,;12.53,-2.03,;11.2,-1.26,;9.85,-2.04,;8.52,-1.27,;7.19,-2.04,;5.86,-1.27,;7.19,-3.58,;8.52,-4.35,;8.52,-5.89,;9.87,-3.58,;5.86,-4.36,;5.86,-5.89,;4.53,-6.67,;3.2,-5.9,;3.2,-4.36,;1.87,-3.59,;.52,-4.36,;1.87,-2.05,;.54,-1.28,;3.2,-1.27,;4.53,-2.04,;4.53,-3.59,)| Show InChI InChI=1S/C19H14BrCl2NO3/c20-19-13-3-1-2-12(11(13)4-5-16(19)24)18-14(21)8-10(9-15(18)22)23-7-6-17(25)26/h1-5,8-9,23-24H,6-7H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor alpha 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18913

(2-{3,5-dichloro-4-[3-(3-ethylphenyl)-4-hydroxyphen...)Show SMILES CCc1cccc(c1)-c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)ccc1O Show InChI InChI=1S/C22H18Cl2O4/c1-2-13-4-3-5-15(8-13)17-12-16(6-7-20(17)25)28-22-18(23)9-14(10-19(22)24)11-21(26)27/h3-10,12,25H,2,11H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 16: 884-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.002
BindingDB Entry DOI: 10.7270/Q2BV7DWM |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18913

(2-{3,5-dichloro-4-[3-(3-ethylphenyl)-4-hydroxyphen...)Show SMILES CCc1cccc(c1)-c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)ccc1O Show InChI InChI=1S/C22H18Cl2O4/c1-2-13-4-3-5-15(8-13)17-12-16(6-7-20(17)25)28-22-18(23)9-14(10-19(22)24)11-21(26)27/h3-10,12,25H,2,11H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50171802

(CHEMBL196642 | N-[3,5-Dichloro-4-(5-isopropyl-6-me...)Show SMILES COc1ccc2c(cccc2c1C(C)C)-c1c(Cl)cc(NC(=O)CC(O)=O)cc1Cl |(1.71,1.66,;1.71,.12,;3.04,-.65,;4.37,.13,;5.72,-.64,;5.72,-2.19,;7.05,-2.96,;7.05,-4.49,;5.7,-5.27,;4.37,-4.5,;4.37,-2.96,;3.04,-2.19,;1.71,-2.96,;.38,-2.19,;1.71,-4.5,;8.38,-2.18,;9.71,-2.95,;9.71,-4.49,;11.04,-2.18,;11.04,-.64,;12.37,.14,;13.7,-.63,;15.05,.16,;13.72,-2.17,;15.06,-2.92,;15.06,-4.46,;16.39,-2.15,;9.71,.13,;8.38,-.64,;7.05,.13,)| Show InChI InChI=1S/C23H21Cl2NO4/c1-12(2)22-15-5-4-6-16(14(15)7-8-19(22)30-3)23-17(24)9-13(10-18(23)25)26-20(27)11-21(28)29/h4-10,12H,11H2,1-3H3,(H,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor alpha 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
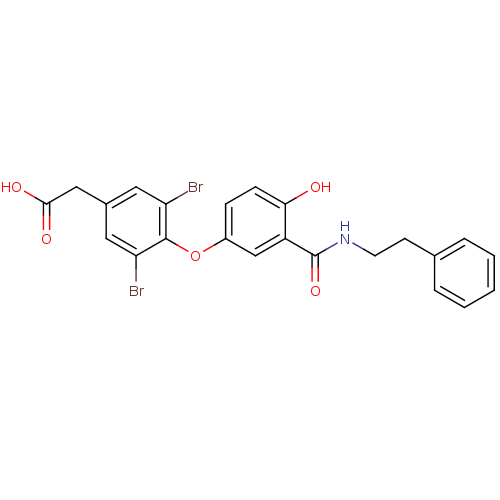
(Homo sapiens (Human)) | BDBM18948
(2-(3,5-dibromo-4-{4-hydroxy-3-[(2-phenylethyl)carb...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCCc2ccccc2)c(Br)c1 Show InChI InChI=1S/C23H19Br2NO5/c24-18-10-15(12-21(28)29)11-19(25)22(18)31-16-6-7-20(27)17(13-16)23(30)26-9-8-14-4-2-1-3-5-14/h1-7,10-11,13,27H,8-9,12H2,(H,26,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18948

(2-(3,5-dibromo-4-{4-hydroxy-3-[(2-phenylethyl)carb...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCCc2ccccc2)c(Br)c1 Show InChI InChI=1S/C23H19Br2NO5/c24-18-10-15(12-21(28)29)11-19(25)22(18)31-16-6-7-20(27)17(13-16)23(30)26-9-8-14-4-2-1-3-5-14/h1-7,10-11,13,27H,8-9,12H2,(H,26,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
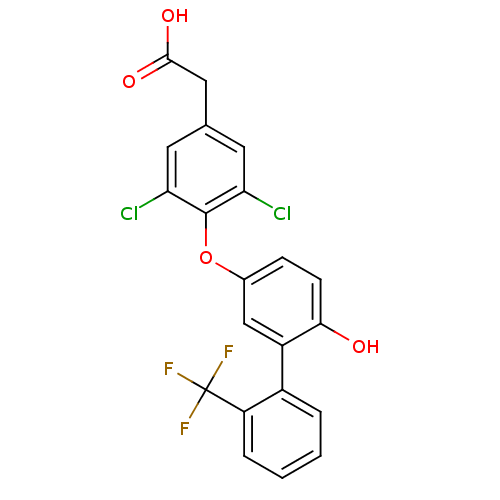
(Homo sapiens (Human)) | BDBM18921
(2-(3,5-dichloro-4-{4-hydroxy-3-[2-(trifluoromethyl...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2ccccc2C(F)(F)F)c(Cl)c1 Show InChI InChI=1S/C21H13Cl2F3O4/c22-16-7-11(9-19(28)29)8-17(23)20(16)30-12-5-6-18(27)14(10-12)13-3-1-2-4-15(13)21(24,25)26/h1-8,10,27H,9H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
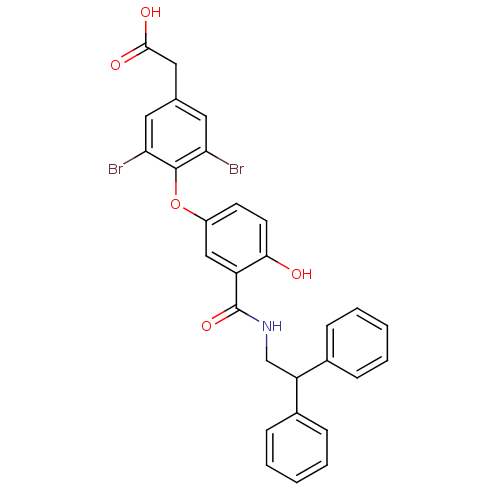
(Homo sapiens (Human)) | BDBM18954
(2-(3,5-dibromo-4-{3-[(2,2-diphenylethyl)carbamoyl]...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCC(c2ccccc2)c2ccccc2)c(Br)c1 Show InChI InChI=1S/C29H23Br2NO5/c30-24-13-18(15-27(34)35)14-25(31)28(24)37-21-11-12-26(33)22(16-21)29(36)32-17-23(19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-14,16,23,33H,15,17H2,(H,32,36)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18921

(2-(3,5-dichloro-4-{4-hydroxy-3-[2-(trifluoromethyl...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2ccccc2C(F)(F)F)c(Cl)c1 Show InChI InChI=1S/C21H13Cl2F3O4/c22-16-7-11(9-19(28)29)8-17(23)20(16)30-12-5-6-18(27)14(10-12)13-3-1-2-4-15(13)21(24,25)26/h1-8,10,27H,9H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18954

(2-(3,5-dibromo-4-{3-[(2,2-diphenylethyl)carbamoyl]...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCC(c2ccccc2)c2ccccc2)c(Br)c1 Show InChI InChI=1S/C29H23Br2NO5/c30-24-13-18(15-27(34)35)14-25(31)28(24)37-21-11-12-26(33)22(16-21)29(36)32-17-23(19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-14,16,23,33H,15,17H2,(H,32,36)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of thyroid hormone receptor alpha |
Bioorg Med Chem 15: 4609-17 (2007)
Article DOI: 10.1016/j.bmc.2007.04.015
BindingDB Entry DOI: 10.7270/Q2W37XJQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50171805

(CHEMBL193864 | [4-(5-Bromo-6-hydroxy-naphthalen-1-...)Show SMILES OC(=O)CNc1cc(Cl)c(c(Cl)c1)-c1cccc2c(Br)c(O)ccc12 |(11.21,-4.35,;12.54,-3.57,;13.87,-4.32,;12.53,-2.03,;11.2,-1.26,;9.85,-2.04,;8.52,-1.27,;7.19,-2.04,;5.86,-1.27,;7.19,-3.58,;8.52,-4.35,;8.52,-5.89,;9.87,-3.58,;5.86,-4.36,;5.86,-5.89,;4.53,-6.67,;3.2,-5.9,;3.2,-4.36,;1.87,-3.59,;.52,-4.36,;1.87,-2.05,;.54,-1.28,;3.2,-1.27,;4.53,-2.04,;4.53,-3.59,)| Show InChI InChI=1S/C18H12BrCl2NO3/c19-18-12-3-1-2-11(10(12)4-5-15(18)23)17-13(20)6-9(7-14(17)21)22-8-16(24)25/h1-7,22-23H,8H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor alpha 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18869
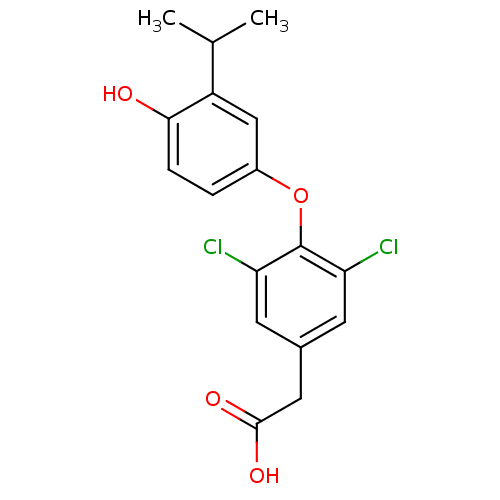
(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18869

(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 16: 884-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.002
BindingDB Entry DOI: 10.7270/Q2BV7DWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18869

(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | 11 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data