

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 282 | n/a | n/a | n/a | 8.0 | n/a |
Genentech, Inc. | Assay Description Specifically, the experiments were conducted in black 384-well polypropylene plates (Corning Glass) in a 15-ul reaction volume consisting of 11 nM HD... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 38.1 | n/a | n/a | n/a | 8.0 | n/a |
Genentech, Inc. | Assay Description Specifically, the experiments were conducted in black 384-well polypropylene plates (Corning Glass) in a 15-ul reaction volume consisting of 11 nM HD... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50276074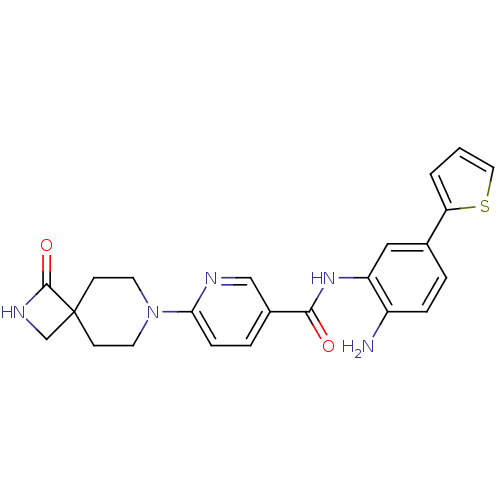 (CHEMBL470791 | HDAC inhibitor, Compound 2 | N-(2-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 24.6 | n/a | n/a | n/a | 8.0 | n/a |
Genentech, Inc. | Assay Description Specifically, the experiments were conducted in black 384-well polypropylene plates (Corning Glass) in a 15-ul reaction volume consisting of 11 nM HD... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 12.1 | n/a | n/a | n/a | 8.0 | n/a |
Genentech, Inc. | Assay Description Specifically, the experiments were conducted in black 384-well polypropylene plates (Corning Glass) in a 15-ul reaction volume consisting of 11 nM HD... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | 163 | n/a | n/a | n/a | 8.0 | n/a |
Genentech, Inc. | Assay Description Specifically, the experiments were conducted in black 384-well polypropylene plates (Corning Glass) in a 15-ul reaction volume consisting of 11 nM HD... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361248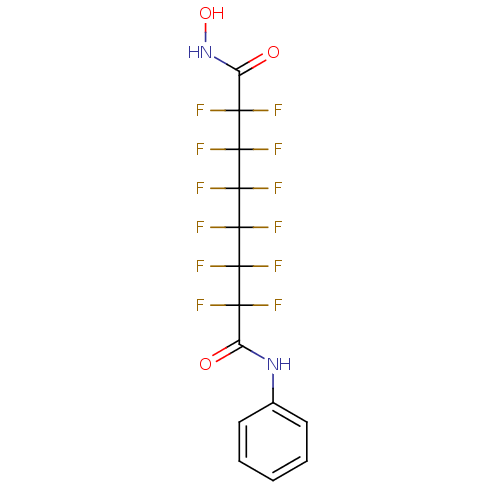 (CHEMBL1934890) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361249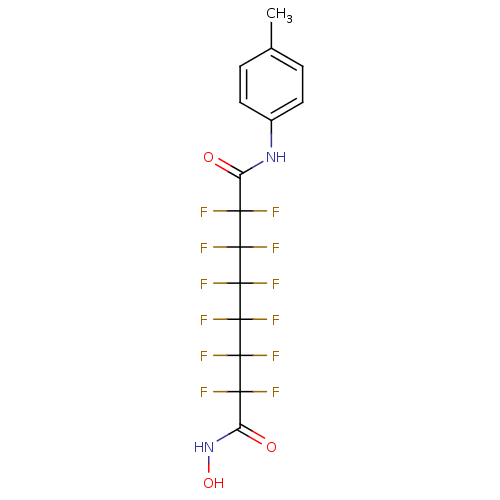 (CHEMBL1934891) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361250 (CHEMBL1934892) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361251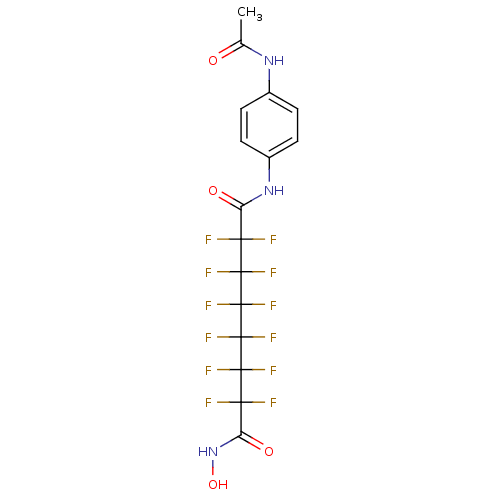 (CHEMBL1934893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361252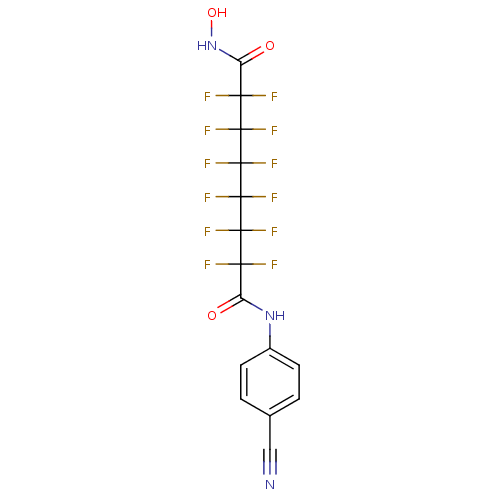 (CHEMBL1934894) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361253 (CHEMBL1934895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361254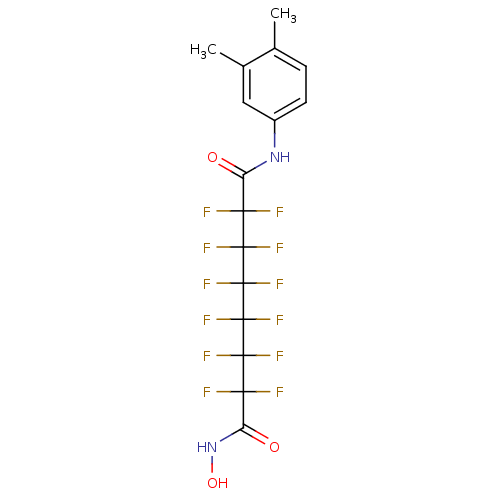 (CHEMBL1934896) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361255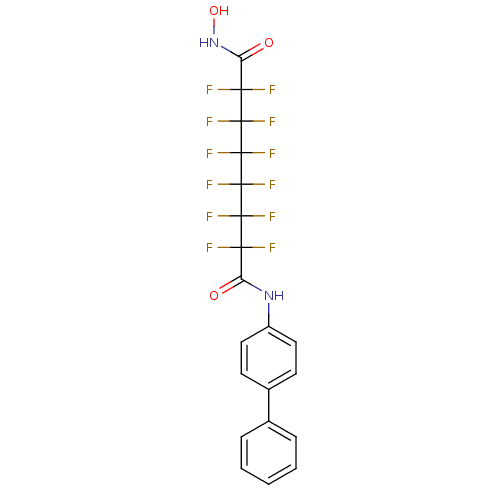 (CHEMBL1934897) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361256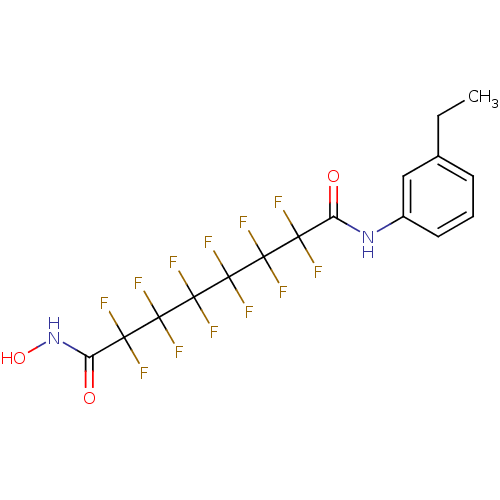 (CHEMBL1934898) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361257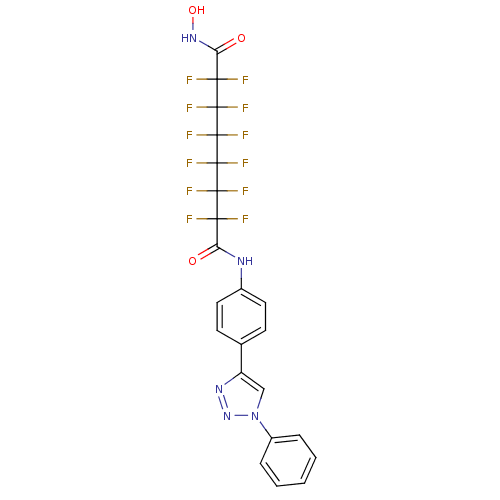 (CHEMBL1934899) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361258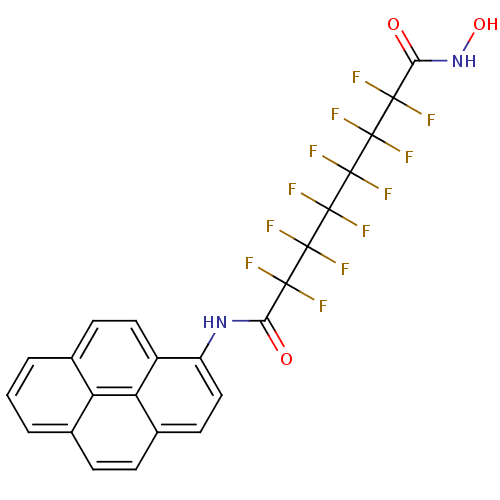 (CHEMBL1934900) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361259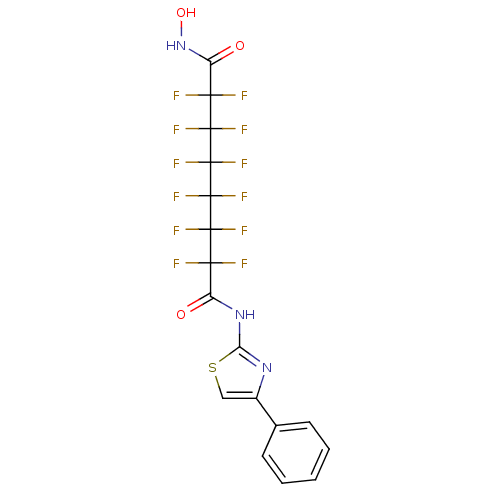 (CHEMBL1934901) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361260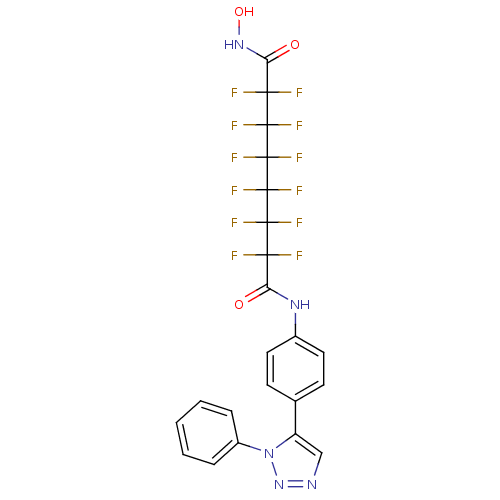 (CHEMBL1934902) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361261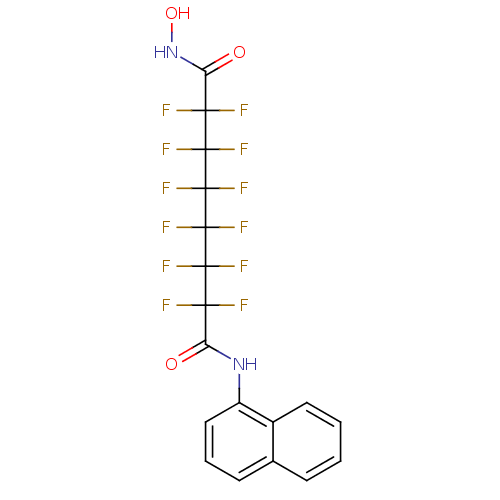 (CHEMBL1934903) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361262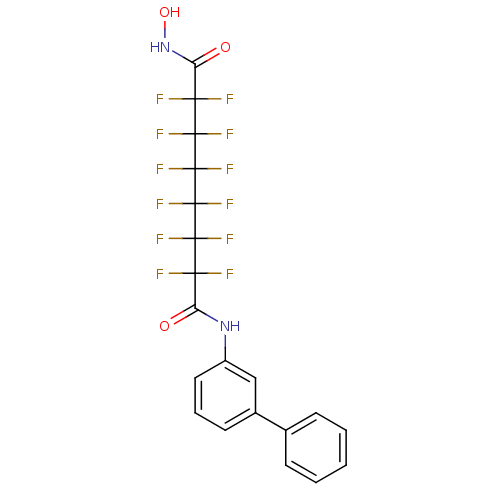 (CHEMBL1934904) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361263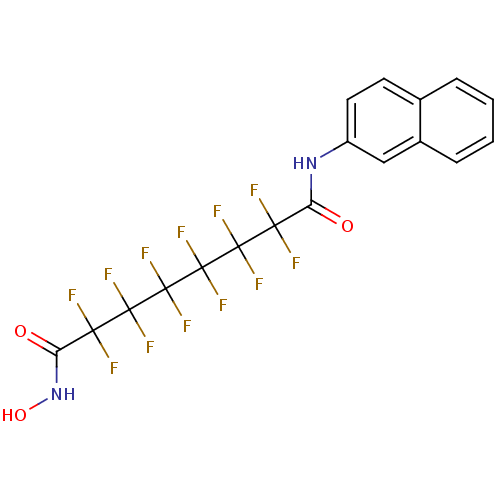 (CHEMBL1934905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361264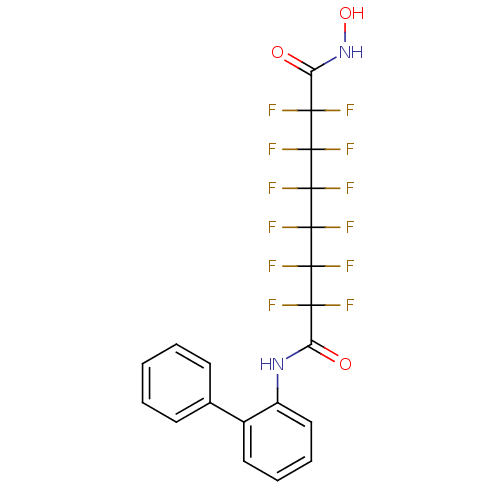 (CHEMBL1934906) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361265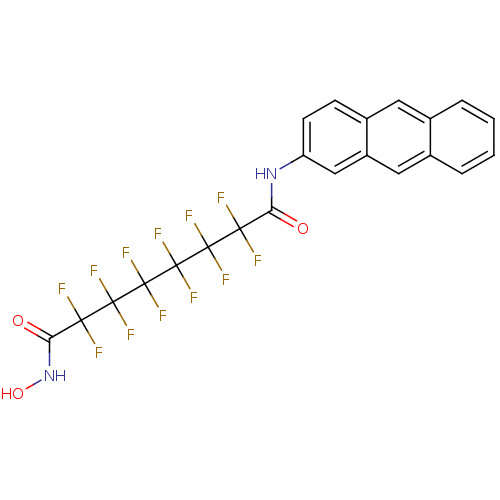 (CHEMBL1934907) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361266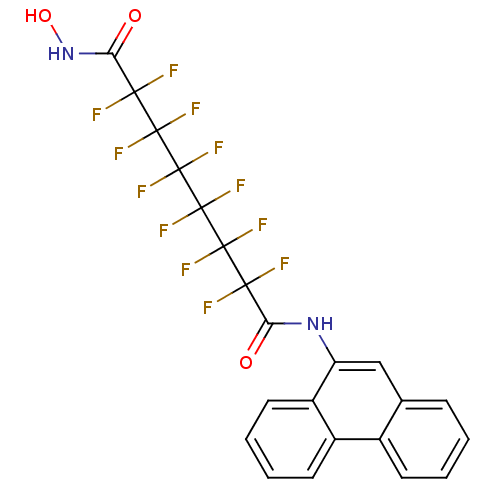 (CHEMBL1934908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361267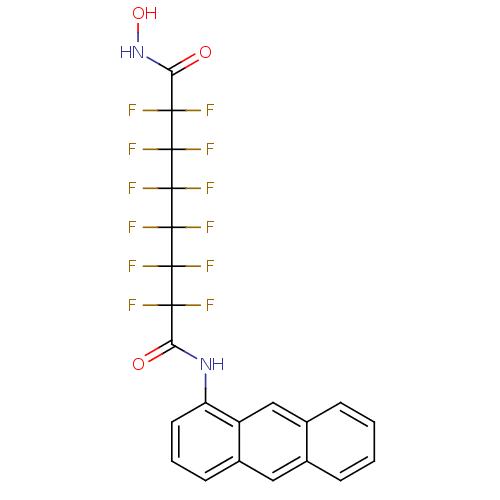 (CHEMBL1934909) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50361268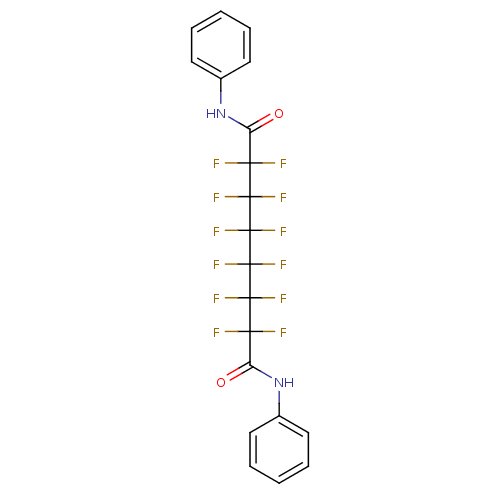 (CHEMBL1934910) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay | Bioorg Med Chem 20: 985-95 (2012) Article DOI: 10.1016/j.bmc.2011.11.041 BindingDB Entry DOI: 10.7270/Q2QJ7HRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413916 (US10421756, Compound 100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413917 (US10421756, Compound 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413918 (US10421756, Compound 102) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413919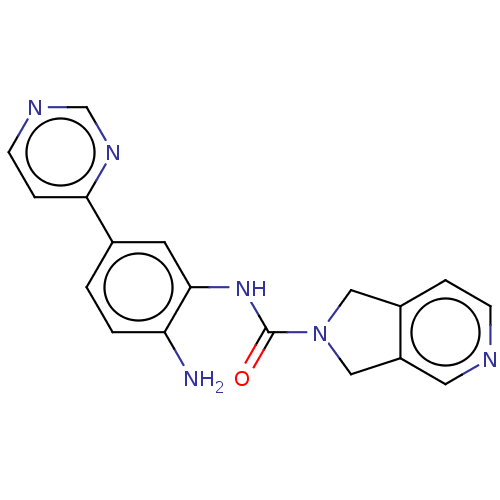 (US10421756, Compound 103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413920 (US10421756, Compound 104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413921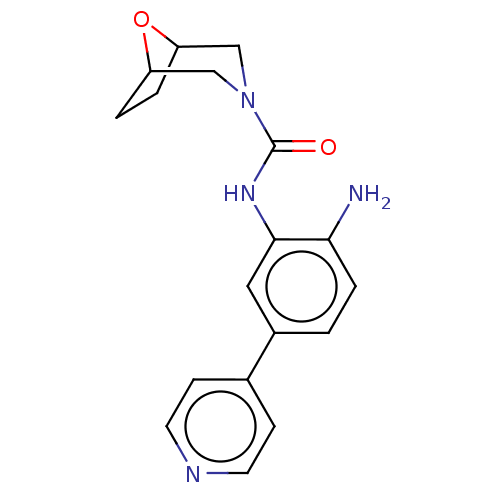 (US10421756, Compound 105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413922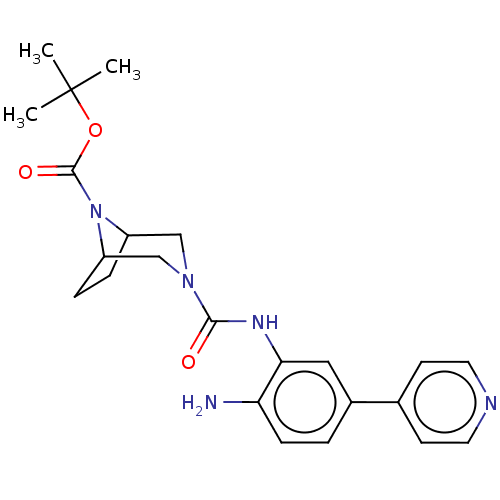 (US10421756, Compound 106 | US10421756, Compound 11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413923 (US10421756, Compound 107 | US10421756, Compound 10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413924 (US10421756, Compound 108) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413923 (US10421756, Compound 107 | US10421756, Compound 10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413922 (US10421756, Compound 106 | US10421756, Compound 11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413927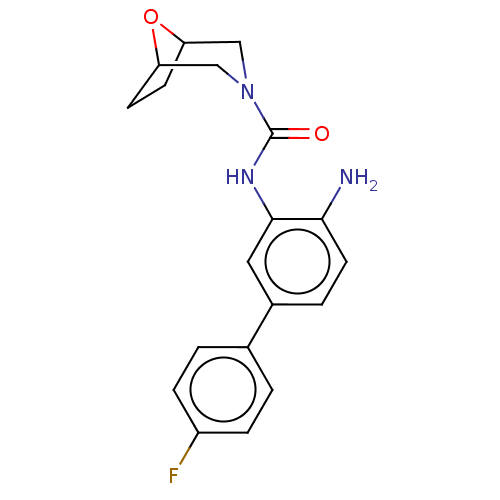 (US10421756, Compound 111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413928 (US10421756, Compound 112) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413929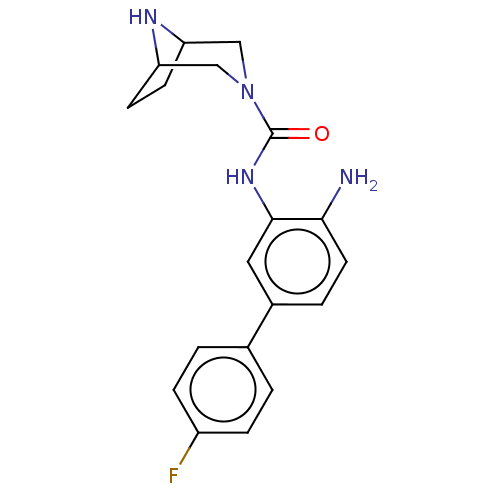 (US10421756, Compound 113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413930 (US10421756, Compound 114) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413931 (US10421756, Compound 115) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413932 (US10421756, Compound 116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413933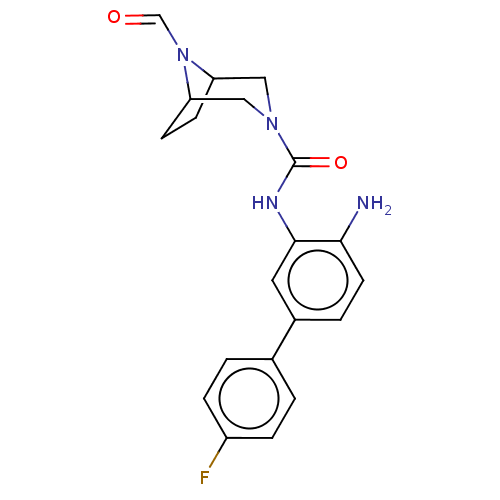 (US10421756, Compound 117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413934 (US10421756, Compound 118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413935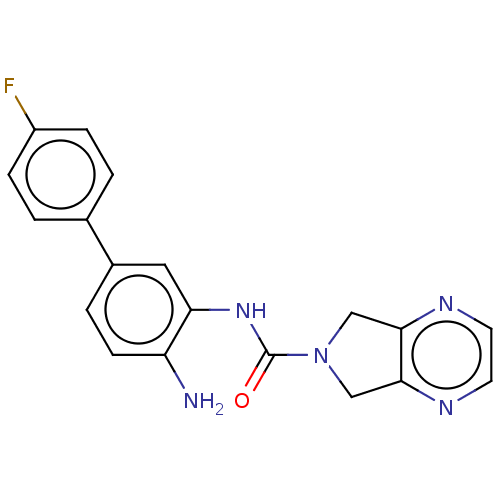 (US10421756, Compound 119) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413936 (US10421756, Compound 120) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413937 (US10421756, Compound 121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM413938 (US10421756, Compound 122) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a |
Rodin Therapeutics, Inc. US Patent | Assay Description HDAC2 containing a C-terminal HIS-Tag (Proteros) and fluorescently-labeled anti-HIS-antibody are diluted in one vial in assay buffer 50 mM Tris, pH 8... | US Patent US10421756 (2019) BindingDB Entry DOI: 10.7270/Q2PK0JGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 129 total ) | Next | Last >> |