 Found 136 hits of ki data for polymerid = 1996
Found 136 hits of ki data for polymerid = 1996 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347454
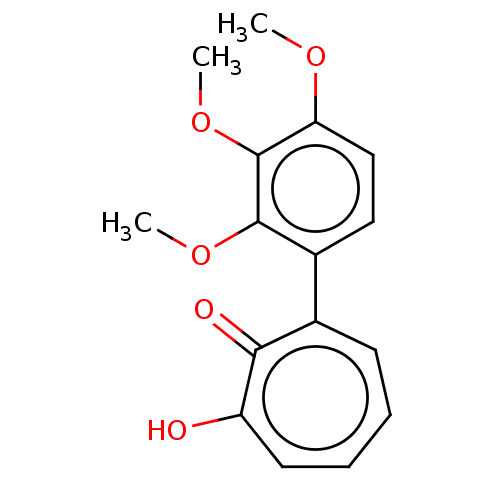
(MO-OH-TM | US9790158, 5)Show InChI InChI=1S/C16H16O5/c1-19-13-9-8-11(15(20-2)16(13)21-3)10-6-4-5-7-12(17)14(10)18/h4-9H,1-3H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50123957
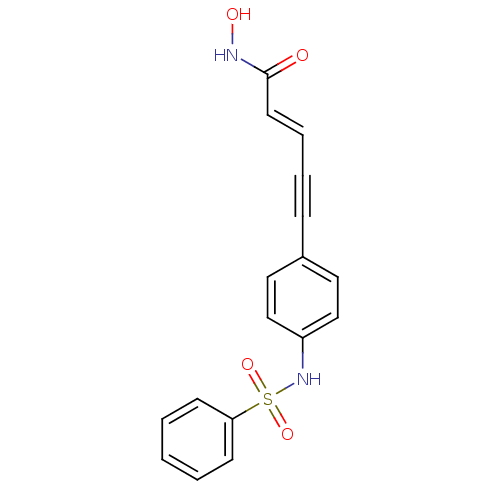
((E)-5-(3-Benzenesulfonylamino-phenyl)-pent-2-en-4-...)Show SMILES ONC(=O)\C=C\C#Cc1ccc(NS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C17H14N2O4S/c20-17(18-21)9-5-4-6-14-10-12-15(13-11-14)19-24(22,23)16-7-2-1-3-8-16/h1-3,5,7-13,19,21H,(H,18,20)/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347452
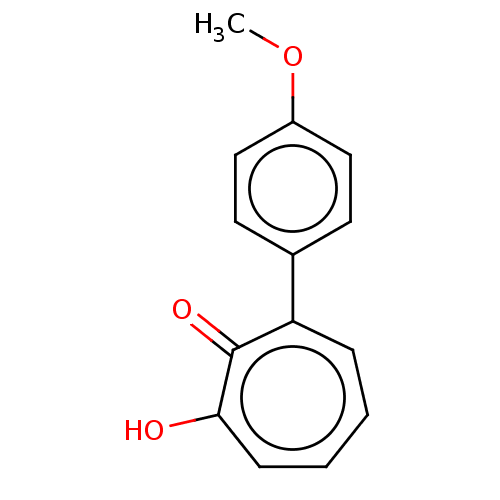
(MO-OH-SM | US9790158, 3)Show InChI InChI=1S/C14H12O3/c1-17-11-8-6-10(7-9-11)12-4-2-3-5-13(15)14(12)16/h2-9H,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347330
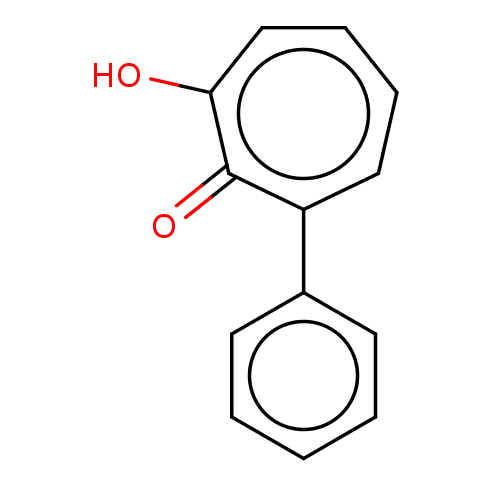
(MO-OH-PHE | US9790158, 1)Show InChI InChI=1S/C13H10O2/c14-12-9-5-4-8-11(13(12)15)10-6-2-1-3-7-10/h1-9H,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347453
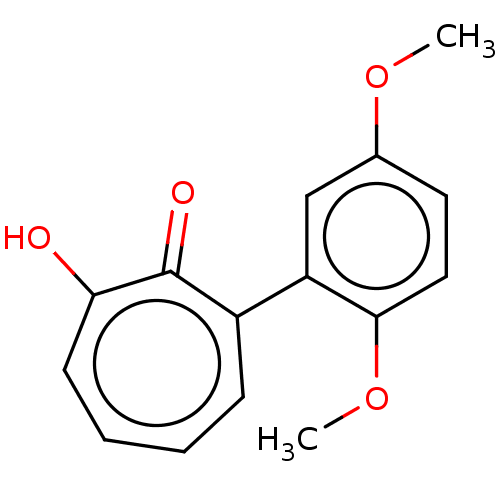
(MO-OH-DM | US9790158, 4)Show InChI InChI=1S/C15H14O4/c1-18-10-7-8-14(19-2)12(9-10)11-5-3-4-6-13(16)15(11)17/h3-9H,1-2H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347330

(MO-OH-PHE | US9790158, 1)Show InChI InChI=1S/C13H10O2/c14-12-9-5-4-8-11(13(12)15)10-6-2-1-3-7-10/h1-9H,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347330

(MO-OH-PHE | US9790158, 1)Show InChI InChI=1S/C13H10O2/c14-12-9-5-4-8-11(13(12)15)10-6-2-1-3-7-10/h1-9H,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347456
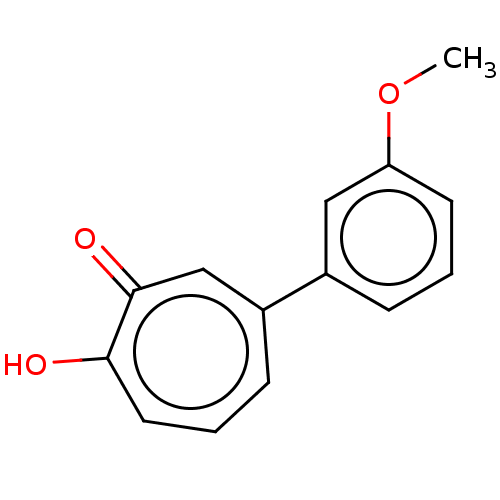
(US9790158, 7)Show InChI InChI=1S/C14H12O3/c1-17-12-6-2-4-10(8-12)11-5-3-7-13(15)14(16)9-11/h2-9H,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347457
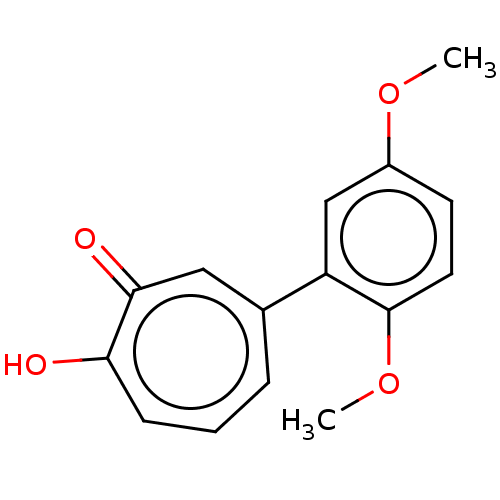
(US9790158, 8)Show InChI InChI=1S/C15H14O4/c1-18-11-6-7-15(19-2)12(9-11)10-4-3-5-13(16)14(17)8-10/h3-9H,1-2H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50492541
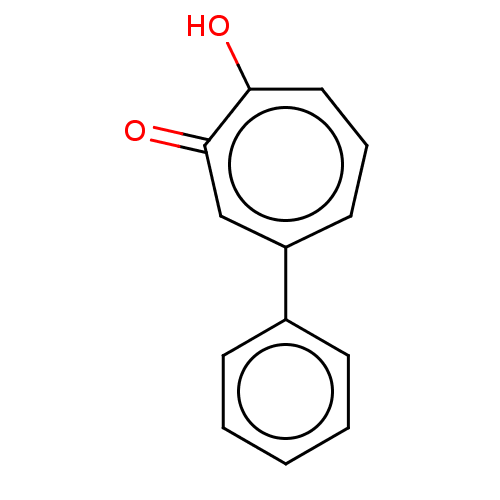
(CHEMBL2408242)Show InChI InChI=1S/C13H10O2/c14-12-8-4-7-11(9-13(12)15)10-5-2-1-3-6-10/h1-9H,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347460
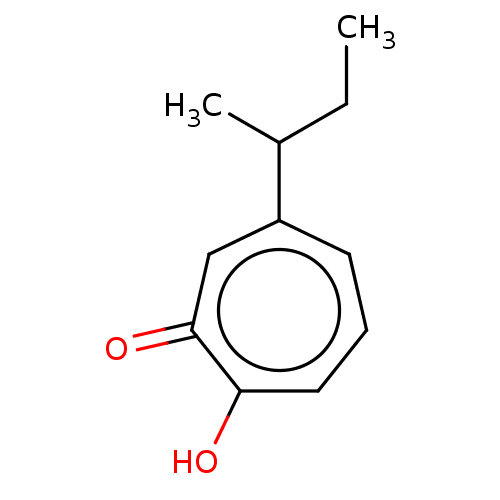
(US9790158, 11)Show InChI InChI=1S/C11H14O2/c1-3-8(2)9-5-4-6-10(12)11(13)7-9/h4-8H,3H2,1-2H3,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50492540

(CHEMBL2408243)Show InChI InChI=1S/C14H12O3/c1-17-12-7-5-10(6-8-12)11-3-2-4-13(15)14(16)9-11/h2-9H,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM348884
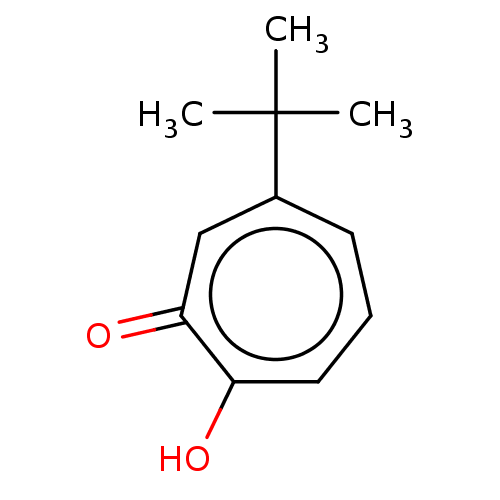
(US9790158, 10)Show InChI InChI=1S/C11H14O2/c1-11(2,3)8-5-4-6-9(12)10(13)7-8/h4-7H,1-3H3,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50210141
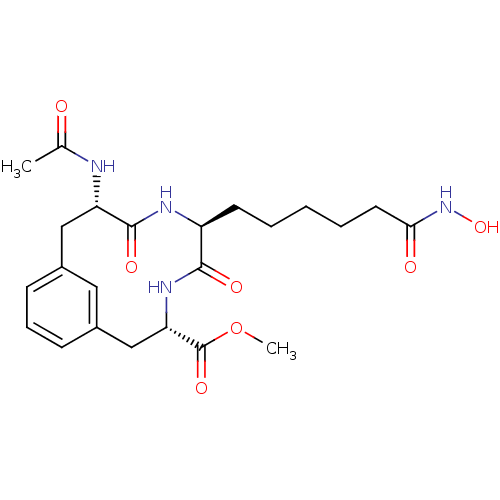
(CHEMBL388195 | methyl (3S,6S,9S)-9-acetamido-6-[5-...)Show SMILES COC(=O)[C@@H]1Cc2cccc(C[C@H](NC(C)=O)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N1)c2 Show InChI InChI=1S/C23H32N4O7/c1-14(28)24-18-12-15-7-6-8-16(11-15)13-19(23(32)34-2)26-21(30)17(25-22(18)31)9-4-3-5-10-20(29)27-33/h6-8,11,17-19,33H,3-5,9-10,12-13H2,1-2H3,(H,24,28)(H,25,31)(H,26,30)(H,27,29)/t17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
J Med Chem 50: 2003-6 (2007)
Article DOI: 10.1021/jm061082q
BindingDB Entry DOI: 10.7270/Q26M36H1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50602203
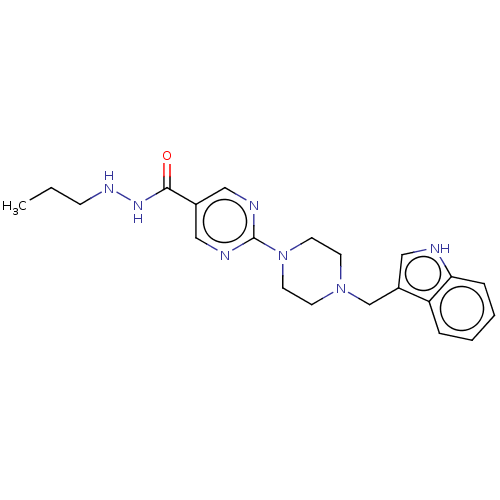
(CHEMBL5171086)Show SMILES CCCNNC(=O)c1cnc(nc1)N1CCN(Cc2c[nH]c3ccccc23)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01132
BindingDB Entry DOI: 10.7270/Q2959NMG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347451
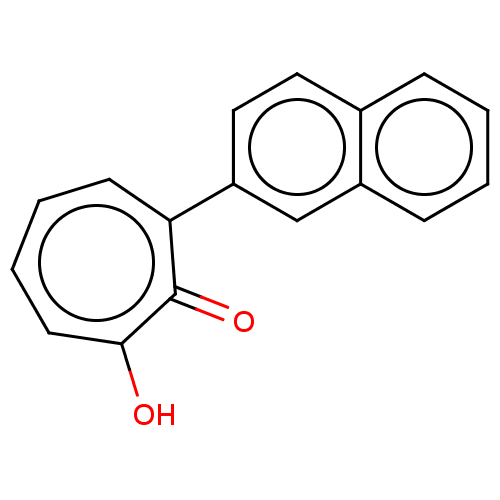
(MO-OH-NAP | US9790158, 2)Show InChI InChI=1S/C17H12O2/c18-16-8-4-3-7-15(17(16)19)14-10-9-12-5-1-2-6-13(12)11-14/h1-11H,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 7.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50492542

(CHEMBL2408248)Show InChI InChI=1S/C11H14O2/c1-8(2)9-5-4-6-11(13-3)10(12)7-9/h4-8H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347460

(US9790158, 11)Show InChI InChI=1S/C11H14O2/c1-3-8(2)9-5-4-6-10(12)11(13)7-9/h4-8H,3H2,1-2H3,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 10.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM348884

(US9790158, 10)Show InChI InChI=1S/C11H14O2/c1-11(2,3)8-5-4-6-9(12)10(13)7-8/h4-7H,1-3H3,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50263458

(CHEMBL4085143)Show SMILES CN1C(=O)C2CN(Cc3c2c2ccccc2n3Cc2cccc(\C=C\C(=O)NO)c2)C1=O Show InChI InChI=1S/C24H22N4O4/c1-26-23(30)18-13-27(24(26)31)14-20-22(18)17-7-2-3-8-19(17)28(20)12-16-6-4-5-15(11-16)9-10-21(29)25-32/h2-11,18,32H,12-14H2,1H3,(H,25,29)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged HDAC8 (1 to 377 residues) expressed in baculovirus-infected insect cells using RHK(Ac)K(Ac)AMC ... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50353227
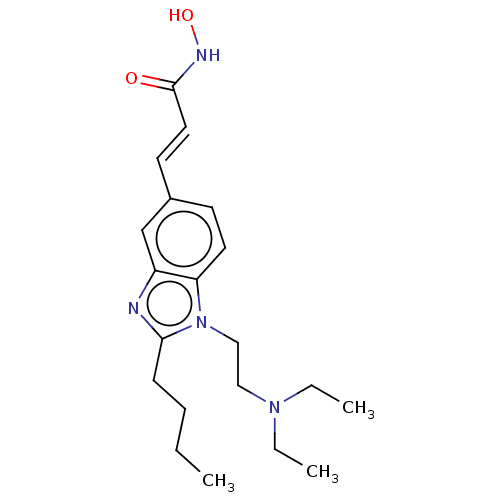
(CHEMBL3215861)Show SMILES Cl.Cl.CCCCc1nc2cc(\C=C\C(=O)NO)ccc2n1CCN(CC)CC Show InChI InChI=1S/C20H30N4O2/c1-4-7-8-19-21-17-15-16(10-12-20(25)22-26)9-11-18(17)24(19)14-13-23(5-2)6-3/h9-12,15,26H,4-8,13-14H2,1-3H3,(H,22,25)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM348883
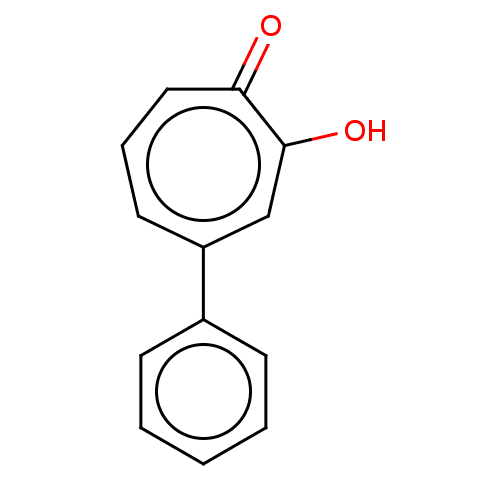
(US9790158, 6)Show InChI InChI=1S/C13H10O2/c14-12-8-4-7-11(9-13(12)15)10-5-2-1-3-6-10/h1-9H,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 19.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM25150
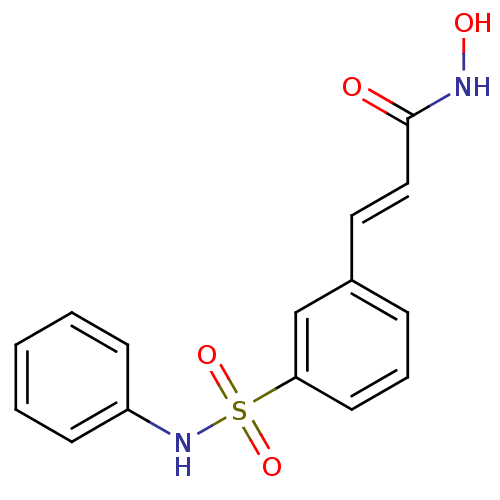
((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347461
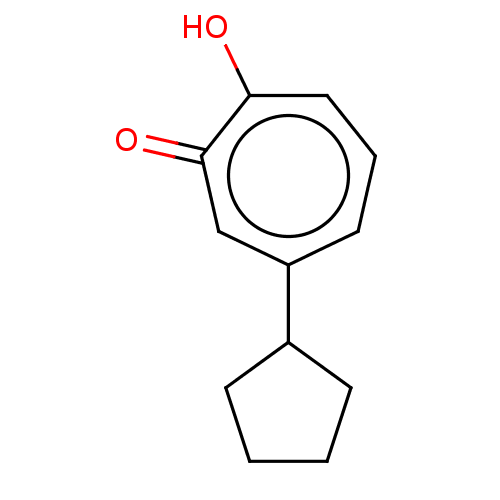
(US9790158, 12)Show InChI InChI=1S/C12H14O2/c13-11-7-3-6-10(8-12(11)14)9-4-1-2-5-9/h3,6-9H,1-2,4-5H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using fluorogenic HDAC substrate after 15 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50602192
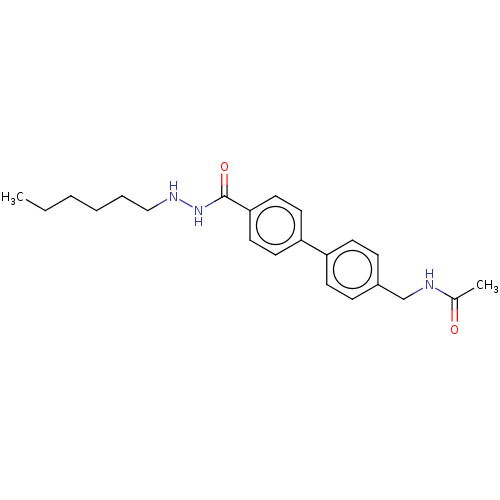
(CHEMBL5174998) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01132
BindingDB Entry DOI: 10.7270/Q2959NMG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50105329
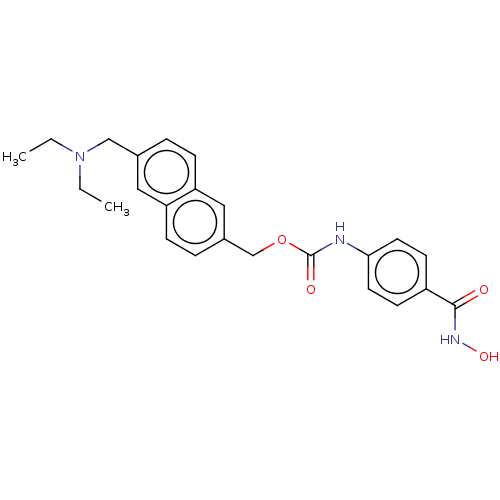
(CHEMBL1213492)Show SMILES CCN(CC)Cc1ccc2cc(COC(=O)Nc3ccc(cc3)C(=O)NO)ccc2c1 Show InChI InChI=1S/C24H27N3O4/c1-3-27(4-2)15-17-5-7-21-14-18(6-8-20(21)13-17)16-31-24(29)25-22-11-9-19(10-12-22)23(28)26-30/h5-14,30H,3-4,15-16H2,1-2H3,(H,25,29)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50263451
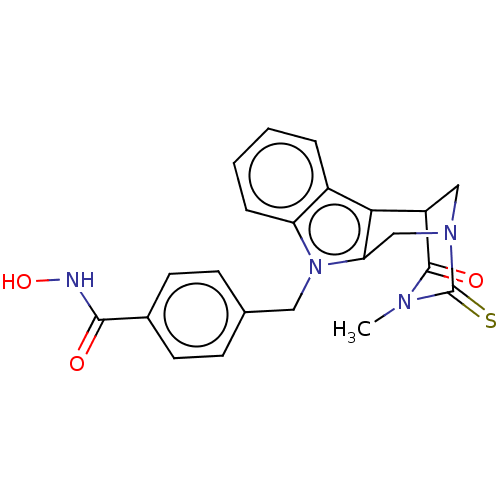
(CHEMBL4065283)Show SMILES CN1C(=O)C2CN(Cc3c2c2ccccc2n3Cc2ccc(cc2)C(=O)NO)C1=S Show InChI InChI=1S/C22H20N4O3S/c1-24-21(28)16-11-25(22(24)30)12-18-19(16)15-4-2-3-5-17(15)26(18)10-13-6-8-14(9-7-13)20(27)23-29/h2-9,16,29H,10-12H2,1H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged HDAC8 (1 to 377 residues) expressed in baculovirus-infected insect cells using RHK(Ac)K(Ac)AMC ... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Catania
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human recombinant full-length HDAC8 expressed in baculovirus expression system assessed as reduction in 7-amino-4... |
Eur J Med Chem 141: 188-196 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.075
BindingDB Entry DOI: 10.7270/Q2QZ2DGS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50105330
(CHEMBL1851943)Show InChI InChI=1S/C20H30N4O2/c1-4-7-8-19-21-17-15-16(10-12-20(25)22-26)9-11-18(17)24(19)14-13-23(5-2)6-3/h9-12,15,26H,4-8,13-14H2,1-3H3,(H,22,25)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50353230
(CHEMBL1830420)Show InChI InChI=1S/C20H30N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h9-12,15,21,26H,3-8,13-14H2,1-2H3,(H,23,25)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50353233
(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50353231
(CHEMBL1830422)Show InChI InChI=1S/C21H32N4O2/c1-4-5-6-7-8-20-23-18-15-17(10-12-21(26)24-27)9-11-19(18)25(20)14-13-22-16(2)3/h9-12,15-16,22,27H,4-8,13-14H2,1-3H3,(H,24,26)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50353229
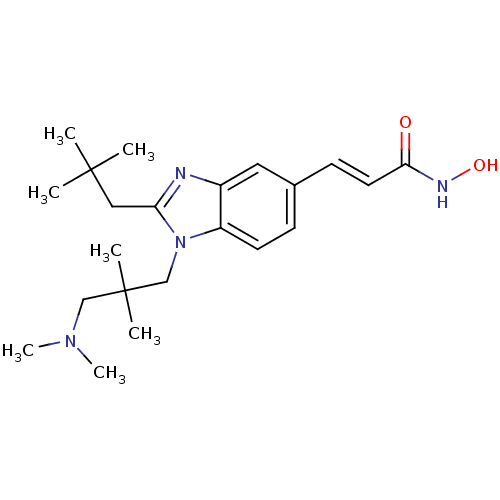
(CHEMBL1830397)Show SMILES CN(C)CC(C)(C)Cn1c(CC(C)(C)C)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C22H34N4O2/c1-21(2,3)13-19-23-17-12-16(9-11-20(27)24-28)8-10-18(17)26(19)15-22(4,5)14-25(6)7/h8-12,28H,13-15H2,1-7H3,(H,24,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50353228
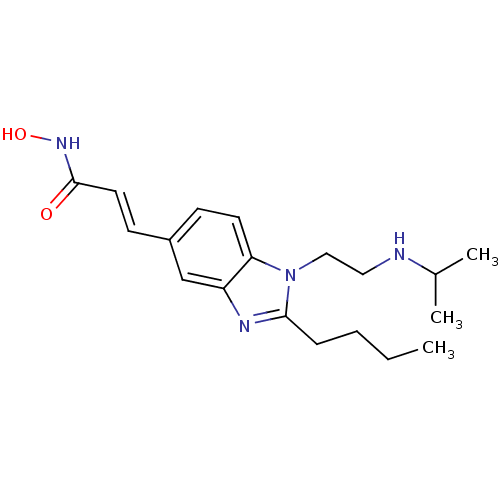
(CHEMBL1830396)Show InChI InChI=1S/C19H28N4O2/c1-4-5-6-18-21-16-13-15(8-10-19(24)22-25)7-9-17(16)23(18)12-11-20-14(2)3/h7-10,13-14,20,25H,4-6,11-12H2,1-3H3,(H,22,24)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50248476
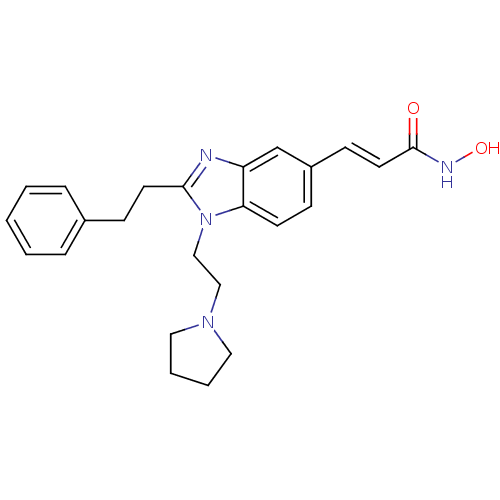
(CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...)Show SMILES ONC(=O)\C=C\c1ccc2n(CCN3CCCC3)c(CCc3ccccc3)nc2c1 Show InChI InChI=1S/C24H28N4O2/c29-24(26-30)13-10-20-8-11-22-21(18-20)25-23(12-9-19-6-2-1-3-7-19)28(22)17-16-27-14-4-5-15-27/h1-3,6-8,10-11,13,18,30H,4-5,9,12,14-17H2,(H,26,29)/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HDAC8 by fluorimetric assay |
Bioorg Med Chem Lett 19: 1403-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.041
BindingDB Entry DOI: 10.7270/Q2FT8KX3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM347452

(MO-OH-SM | US9790158, 3)Show InChI InChI=1S/C14H12O3/c1-17-11-8-6-10(7-9-11)12-4-2-3-5-13(15)14(12)16/h2-9H,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50263447
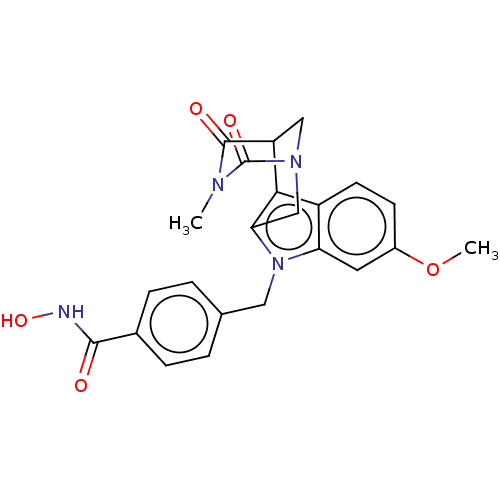
(CHEMBL4062190)Show SMILES COc1ccc2c3C4CN(Cc3n(Cc3ccc(cc3)C(=O)NO)c2c1)C(=O)N(C)C4=O Show InChI InChI=1S/C23H22N4O5/c1-25-22(29)17-11-26(23(25)30)12-19-20(17)16-8-7-15(32-2)9-18(16)27(19)10-13-3-5-14(6-4-13)21(28)24-31/h3-9,17,31H,10-12H2,1-2H3,(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged HDAC8 (1 to 377 residues) expressed in baculovirus-infected insect cells using RHK(Ac)K(Ac)AMC ... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50353234
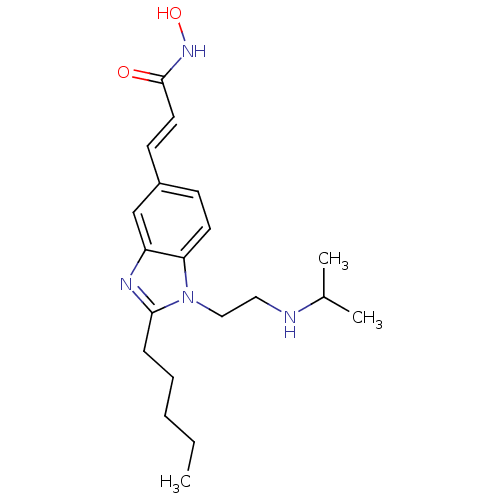
(CHEMBL1830537)Show InChI InChI=1S/C20H30N4O2/c1-4-5-6-7-19-22-17-14-16(9-11-20(25)23-26)8-10-18(17)24(19)13-12-21-15(2)3/h8-11,14-15,21,26H,4-7,12-13H2,1-3H3,(H,23,25)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50248476

(CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...)Show SMILES ONC(=O)\C=C\c1ccc2n(CCN3CCCC3)c(CCc3ccccc3)nc2c1 Show InChI InChI=1S/C24H28N4O2/c29-24(26-30)13-10-20-8-11-22-21(18-20)25-23(12-9-19-6-2-1-3-7-19)28(22)17-16-27-14-4-5-15-27/h1-3,6-8,10-11,13,18,30H,4-5,9,12,14-17H2,(H,26,29)/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC8 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50463739
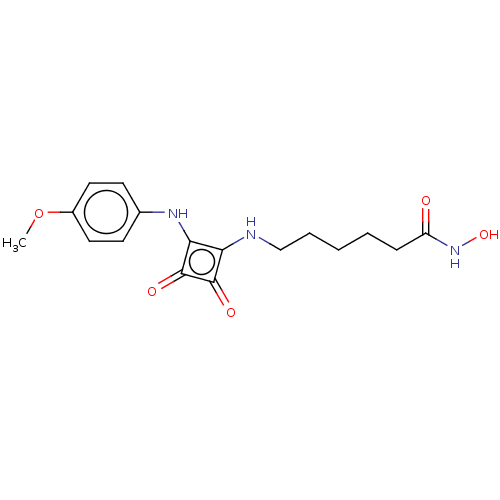
(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using fluorogenic HDAC substrate after 15 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM79632
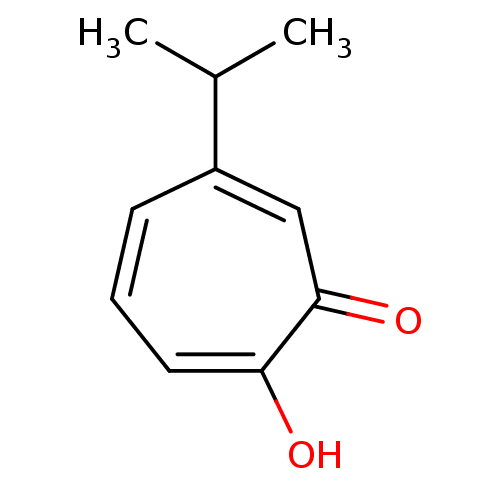
(2-hydroxy-6-isopropyl-cyclohepta-2,4,6-trien-1-one...)Show InChI InChI=1S/C10H12O2/c1-7(2)8-4-3-5-9(11)10(12)6-8/h3-7H,1-2H3,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT
US Patent
| Assay Description
Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... |
US Patent US9790158 (2017)
BindingDB Entry DOI: 10.7270/Q2QZ2D3V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50463743
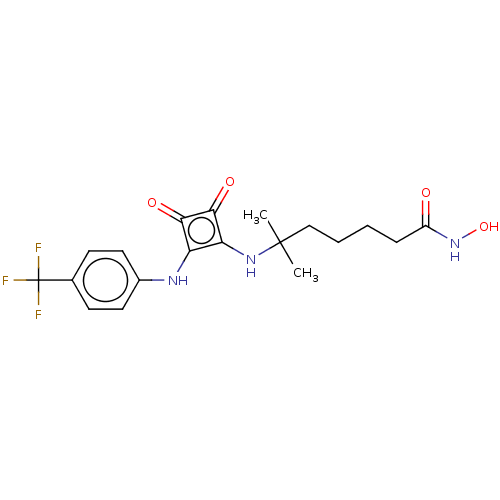
(CHEMBL4241370)Show SMILES CC(C)(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C19H22F3N3O4/c1-18(2,10-4-3-5-13(26)25-29)24-15-14(16(27)17(15)28)23-12-8-6-11(7-9-12)19(20,21)22/h6-9,23-24,29H,3-5,10H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using fluorogenic HDAC substrate after 15 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data